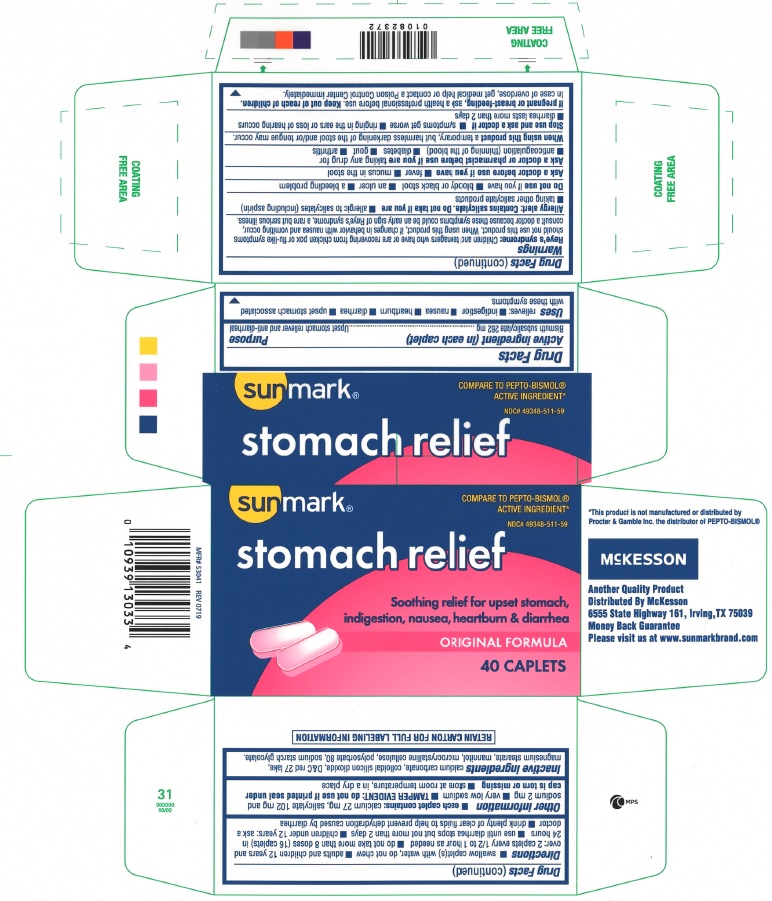
Label: STOMACH RELIEF- bismuth subsalicylate tablet
- NDC Code(s): 49348-511-59
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. when using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert:Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
Do not use if you have
- bloody or black stool
- an ulcer
- a bleeding problem
Ask a doctor before use if you have
- fever
- mucus in the stool
Ask a doctor or pharmacist before use if you are taking any drug for
- anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
-
Directions
- Swallow caplet(s) with water, do not chew
- adults and children 12 years and over: 2 caplets every 1/2 to 1 hour as needed
- do not take more than 8 doses (16 caplets) in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration which may accompany diarrhea.
- Other information
- Inactive ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
STOMACH RELIEF
bismuth subsalicylate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49348-511 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (SALICYLIC ACID - UNII:O414PZ4LPZ, BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C RED NO. 27 (UNII: 2LRS185U6K) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color pink Score no score Shape capsule Size 16mm Flavor Imprint Code G172 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49348-511-59 1 in 1 CARTON 09/27/2019 1 40 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part335 09/27/2019 Labeler - Strategic Sourcing Services LLC (116956644)