CARBAPHEN PED CH- chlophedianol hcl, chlorpheniramine maleate and phenylephrine hcl suspension
Gil Pharmaceutical Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Carbaphen Pediatric
Active ingredient (in each 1 mL)
Chlophedianol HCl 8 mg
Chlorpheniramine Maleate 1.25 mg
Phenylephrine HCl 2.5 mg
Uses
Temporarily relieves:
- cough due to the common cold.
- runny nose and sneezing, itching of the nose or throat, and itchy, watery eyes due to hay fever or other upper respiratory allergies.
- nasal congestion due to the common cold.
Warnings
Do not use
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if you have
- asthma
- emphysema
- glaucoma
- diabetes
- excessive phlegm (mucus)
- breathing problems
- chronic bronchitis
- persistent or chronic cough
- cough associated with smoking
- trouble urinating due to enlarged prostate gland
- a sodium-restricted diet
- thyroid disorders
- heart disease
- high blood pressure
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
Directions
- use 5 mL dosing syringe
- do not exceed 4 doses in 24 hours
| Children 6 to 12 years of age: | 1 to 1.5 mL every 6 to 8 hours. |
| Children under 6 years of age: | Consult a doctor. |
Inactive ingredients
banana flavor, benzoic acid, blueberry flavor, citric acid, ethyl alcohol, glycerin, methylparaben, natural masking agent, propylene glycol, propylparaben, sodium citrate, sorbital, sucralose, water, and xanthan gum.
Package/Label Principal Display Panel
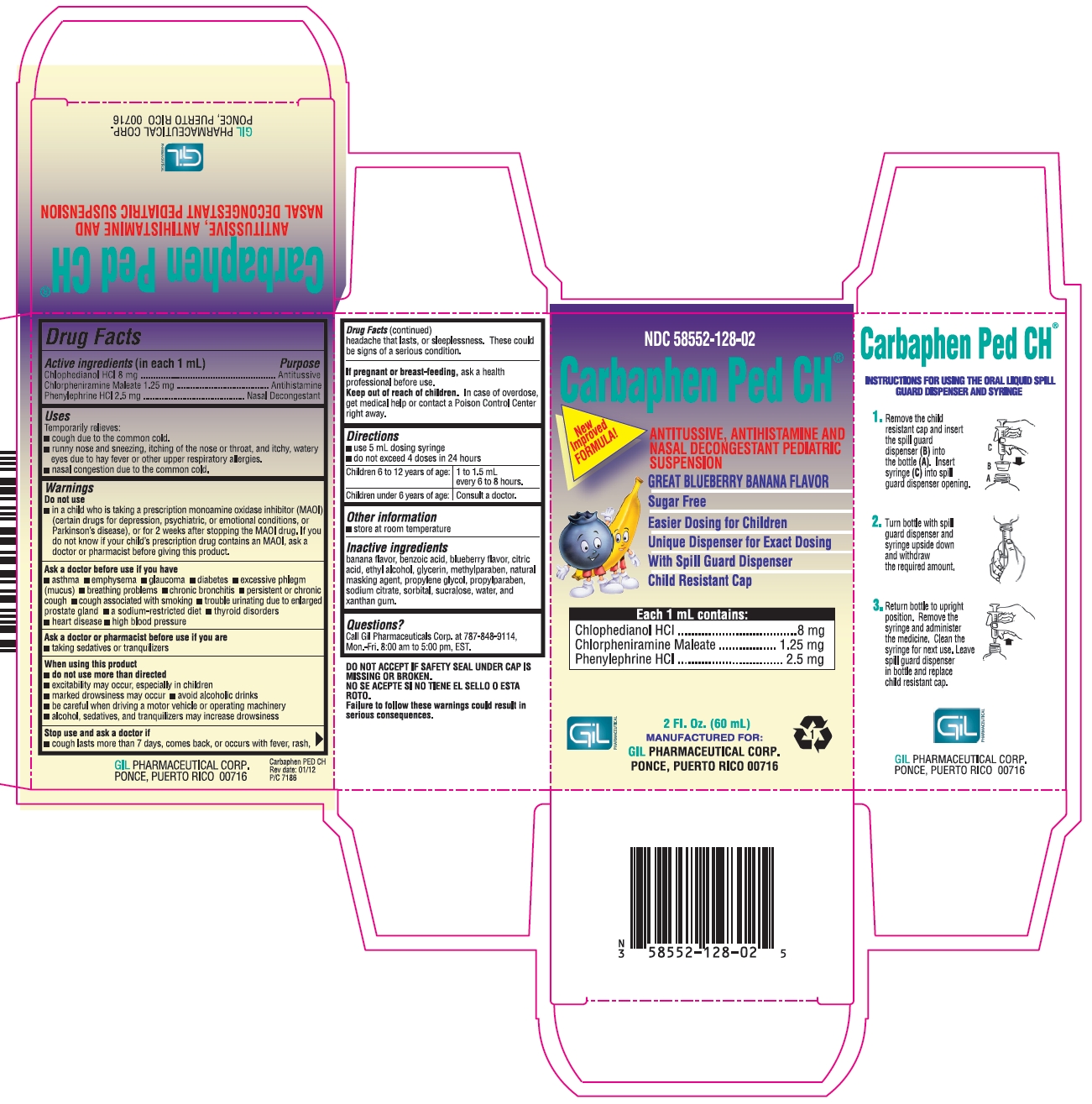
NDC 58552-128-02
Carbapen Ped CH®
New Improved FORMULA!
ANTITUSSIVE, ANTIHISTAMINE AND NASAL DECONGESTANT PEDIATRIC SUSPENSION
GREAT BLUEBERRY BANANA FLAVOR
Sugar Free
Easier Dosing for Children
Unique Dispenser for Exact Dosing
With Spill Guard Dispenser
Child Resistant Cap
Each 1 mL contains:
Chlophedianol HCl...............................8 mg
Chlorpheniramine Maleate...............1.25 mg
Phenylephrine HCl.............................2.5 mg
2 Fl. Oz. (60 mL)
MANUFACTURED FOR:
GIL PHARMACEUTICAL CORP.
PONCE, PUERTO RICO 00716
| CARBAPHEN PED CH
chlophedianol hcl, chlorpheniramine maleate and phenylephrine hcl suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Gil Pharmaceutical Corp (176826592) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nexgen Pharma, Inc. | 806784679 | manufacture(58552-128) | |