STOOL SOFTENER- docusate sodium capsule
Strategic Sourcing Services LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
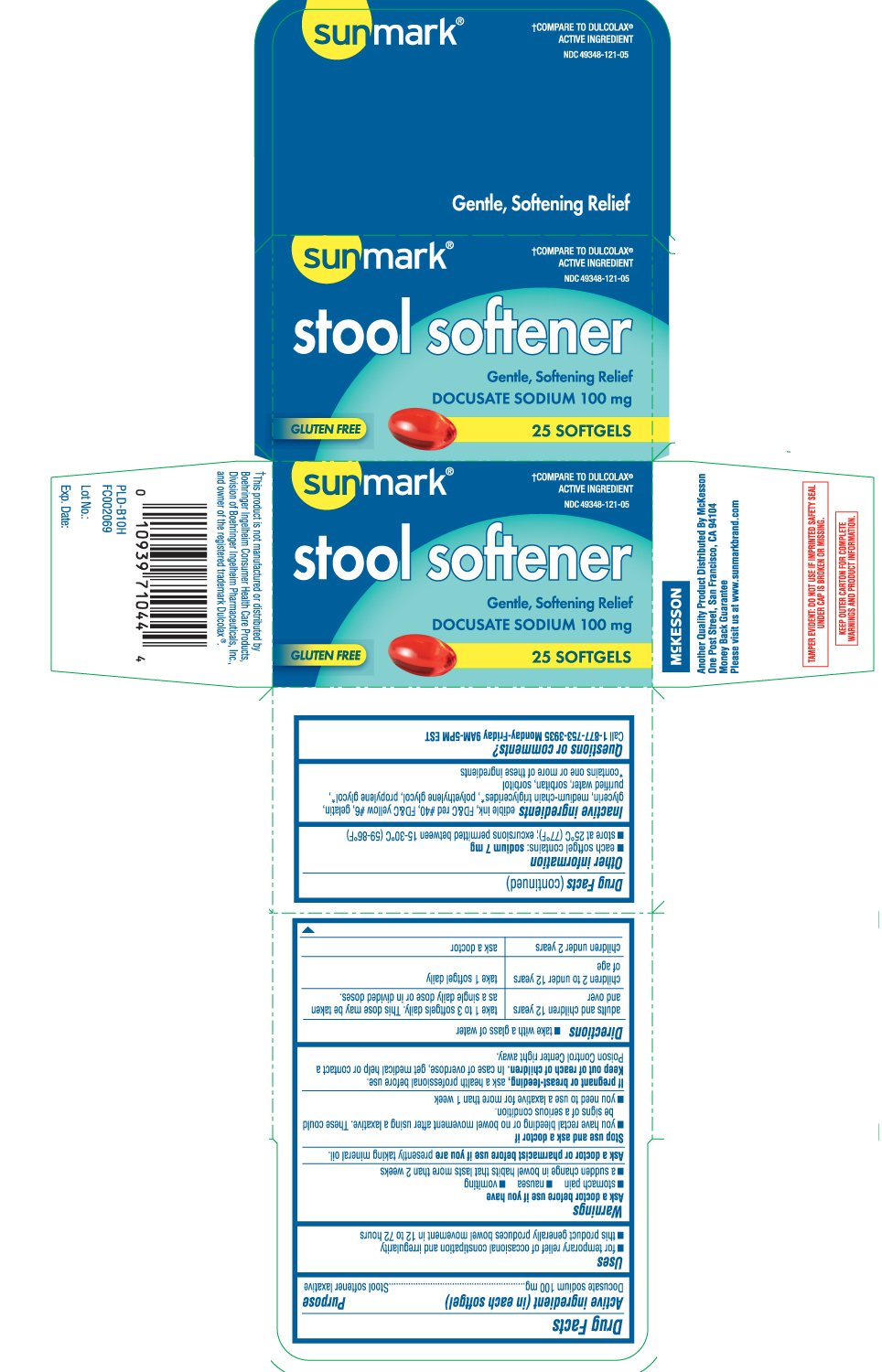
Uses
- for temporary relief of occasional constipation and irregularity
- this product generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
Directions
- take with a glass of water
| adults and children 12 years and over | take 1 to 3 softgels daily. This dose may be taken as a single daily dose or in divided doses. |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years of age | ask a doctor |
Other information
- each softgel contains: sodium 7 mg
- store at 25ºC (77ºF); excursions permitted between 15-30ºC (59-86ºF)
Inactive ingredients
edible ink, FD&C red #40, FD&C yellow #6, gelatin, glycerin, medium-chain triglycerides*, polyethylene glycol, propylene glycol*, purified water, sorbitan, sorbitol
*contains one or more of these ingredients
Principal Display Panel
†Compare to Dulcolax® active ingredient
STOOL SOFTENER
Gentle, Softening Relief
DOCUSATE SODIUM 100 mg
Gluten Free
SOFTGELS
†This product is not manufactured or distributed by Boehringer Ingelheim Consumer Health Care Products, Division of Boehringer Ingelheim Pharmaceuticals, Inc., and owner of the registered trademark Dulcolax®.
Another quality product Distributed by McKesson
One Post Street, San Francisco, CA 94104
Please visit us at www.sunmarkbrand.com
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| STOOL SOFTENER
docusate sodium capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |