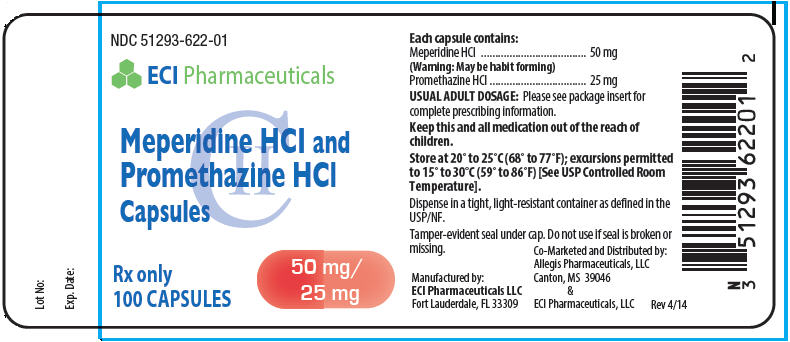
MEPERIDINE HYDROCHLORIDE AND PROMETHAZINE HYDROCHLORIDE- meperidine hydrochloride and promethazine hydrochloride capsule
ECI Pharmaceuticals LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
MEPERIDINE HCl AND PROMETHAZINE HCl -meperidine hydrochloride and promethazine hydrochloride capsule C-II
DESCRIPTION
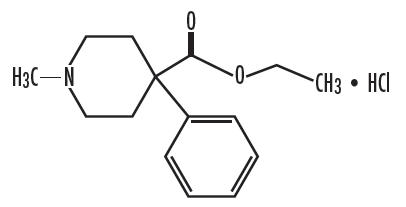
Meperidine HCl and Promethazine HCl capsules each contain 50 mg meperidine hydrochloride and 25 mg promethazine hydrochloride for oral administration. Meperidine hydrochloride is a white crystalline substance with a melting point of 186°C to 189°C. It is readily soluble in water and has a neutral reaction and a slightly bitter taste. Chemically, Meperidine hydrochloride is 4-Piperidinecarboxylic acid, 1-methyl-4-phenyl-, ethyl ester, hydrochloride and has the following structure:
Promethazine hydrochloride occurs as a white to faint yellow, practically odorless, crystalline powder which slowly oxidizes and turns blue on prolonged exposure to air. It is freely soluble in water and soluble in alcohol.
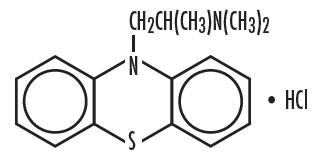
Chemically, Promethazine hydrochloride is (10H-phenothiazine-10-ethanamine, N,N,α-trimethyl-, monohydrochloride, (±)-) is a racemic compound and has the following structural formula:
Inactive Ingredients:
Black ink, FD&C Blue #1, FD&C Red #3, FD&C Yellow #6, gelatin, lactose, magnesium stearate, talc and titanium dioxide.
CLINICAL PHARMACOLOGY
Meperidine hydrochloride is a narcotic analgesic with multiple actions qualitatively similar to those of morphine; the most prominent of these involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value are analgesia and sedation.
There is some evidence which suggests that meperidine may produce less smooth muscle spasm, constipation, and depression of the cough reflex than equianalgesic doses of morphine.
Promethazine is a phenothiazine derivative which differs structurally from the antipsychotic phenothiazines by the presence of a branched side chain and no ring substitution. It is thought that this configuration is responsible for its relative lack (1/10 that of chlorpromazine) of dopamine antagonist properties.
Promethazine is an H1 receptor-blocking agent. In addition to its antihistaminic action, it provides clinically useful sedative and antiemetic effects.
Promethazine is well absorbed from the gastrointestinal tract. Clinical effects are apparent within 20 minutes after oral administration and generally last 4 to 6 hours, although they may persist as long as 12 hours. Promethazine is metabolized by the liver to a variety of compounds; the sulfoxides of promethazine and N-demethylpromethazine are the predominant metabolites appearing in the urine.
| INDICATIONS |
| Based on a review of this drug by the National Academy of Sciences - National Research Council and/or other information, FDA has classified the indications as follows: |
| Possibly Effective: As analgesia for moderate to moderately severe pain. |
CONTRAINDICATIONS
Hypersensitivity to meperidine or promethazine.
Meperidine is contraindicated in patients who are receiving monoamine oxidase inhibitors (MAOI) or those who have received such agents within 14 days. Therapeutic doses of meperidine have inconsistently precipitated unpredictable, severe, and occasionally fatal reactions in patients who have received such agents within 14 days. The mechanism of these reactions is unclear. Some have been characterized by coma, severe respiratory depression, cyanosis, and hypotension and have resembled the syndrome of acute narcotic overdose. In other reactions the predominant manifestations have been hyperexcitability, convulsions, tachycardia, hyperpyrexia, and hypertension. Although it is not known that other narcotics are free of the risk of such reaction, virtually all of the reported reactions have occurred with meperidine. If a narcotic is needed in such patients, a sensitivity test should be performed in which repeated, small, incremental doses of morphine are administered over the course of several hours while the patient's condition and vital signs are under careful observation.
(Intravenous hydrocortisone or prednisolone have been used to treat severe reactions, with the addition of intravenous chlorpromazine in those cases exhibiting hypertension and hyperpyrexia. The usefulness and safety of narcotic antagonists in the treatment of these reactions is unknown.)
Promethazine hydrochloride is contraindicated for use in pediatric patients less than two years of age.
Promethazine is contraindicated in comatose states, and in individuals known to be hypersensitive or to have had an idiosyncratic reaction to promethazine or to other phenothiazines.
WARNINGS
WARNING
PROMETHAZINE HYDROCHLORIDE SHOULD NOT BE USED IN PEDIATRIC PATIENTS LESS THAN 2 YEARS OF AGE BECAUSE OF THE POTENTIAL FOR FATAL RESPIRATORY DEPRESSION.
POSTMARKETING CASES OF RESPIRATORY DEPRESSION, INCLUDING FATALITIES, HAVE BEEN REPORTED WITH USE OF PROMETHAZINE HYDROCHLORIDE IN PEDIATRIC PATIENTS LESS THAN 2 YEARS OF AGE. A WIDE RANGE OF WEIGHT-BASED DOSES OF PROMETHAZINE HYDROCHLORIDE HAVE RESULTED IN RESPIRATORY DEPRESSION IN THESE PATIENTS.
CAUTION SHOULD BE EXERCISED WHEN ADMINISTERING PROMETHAZINE HYDROCHLORIDE TO PEDIATRIC PATIENTS 2 YEARS OF AGE AND OLDER.
IT IS RECOMMENDED THAT THE LOWEST EFFECTIVE DOSE OF PROMETHAZINE HYDROCHLORIDE BE USED IN PEDIATRIC PATIENTS 2 YEARS OF AGE AND OLDER AND CONCOMITANT ADMINISTRATION OF OTHER DRUGS WITH RESPIRATORY DEPRESSANT EFFECTS BE AVOIDED.
Interaction with Other Central Nervous System Depressants
Meperidine should be used with great caution and in reduced dosage in patients who are concurrently receiving other narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics, tricyclic antidepressants, and other CNS depressants (including alcohol). Respiratory depression, hypotension, and profound sedation or coma may result.
The sedative action of promethazine hydrochloride is additive to the sedative effects of central nervous system depressants; therefore, agents such as alcohol, barbiturates, and narcotic analgesics should either be eliminated or given in reduced dosage in the presence of promethazine hydrochloride. When given concomitantly with promethazine hydrochloride, the dose of barbiturates should be reduced by at least one-half and the dose of analgesic depressants, such as morphine or meperidine, should be reduced by one-quarter to one-half.
Head Injury and Increased Intracranial Pressure
The respiratory-depressant effects of meperidine and its capacity to elevate cerebrospinal-fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries. In such patients, meperidine must be used with extreme caution and only if its use is deemed essential.
Asthma and Other Respiratory Conditions
Meperidine should be used with extreme caution in patients having an acute asthmatic attack, patients with chronic obstructive pulmonary disease or cor pulmonale, patients having substantially decreased respiratory reserve, and patients with preexisting respiratory depression, hypoxia, or hypercapnia. In such patients, even usual therapeutic doses of narcotics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Hypotensive Effect
The administration of meperidine may result in severe hypotension in an individual whose ability to maintain his blood pressure has already been compromised by a depleted blood volume or concurrent administration of drugs such as the phenothiazines or certain anesthetics.
Usage in Ambulatory Patients
Meperidine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Meperidine, like other narcotics, may produce orthostatic hypotension in ambulatory patients.
Usage in Pregnancy and Lactation
Meperidine should not be used in pregnant women prior to the labor period, unless in the judgment of the physician the potential benefits outweigh the possible hazards, because safe use in pregnancy prior to labor has not been established relative to possible adverse effects on fetal development.
When used as an obstetrical analgesic, meperidine crosses the placental barrier and can produce respiratory depression in the newborn; resuscitation may be required (see OVERDOSAGE).
NURSING MOTHERS
Due to the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential benefits of the drug to the nursing woman. Meperidine appears in the milk of nursing mothers receiving the drug.
PRECAUTIONS
Supraventricular Tachycardias
Meperidine should be used with caution in patients with atrial flutter and other supraventricular tachycardias because of a possible vagolytic action which may produce a significant increase in the ventricular response rate.
Convulsions
Meperidine may aggravate preexisting convulsions in patients with convulsive disorders. If dosage is escalated substantially above recommended levels because of tolerance development, convulsions may occur in individuals without a history of convulsive disorders.
Acute Abdominal Conditions
The administration of meperidine or other narcotics may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Special-Risk Patients
Meperidine should be given with caution, and the initial dose should be reduced in certain patients, such as the elderly or debilitated, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, sickle cell anemia, pheochromocytoma, acute alcoholism, CNS depression or coma; delirium tremens, kyphoscoliosis associated with respiratory depression; myxedema or hypothyroidism, renal function and prostatic hypertrophy or urethral stricture.
Ambulatory patients should be cautioned against driving automobiles or operating dangerous machinery until it is known that they do not become drowsy or dizzy from promethazine hydrochloride therapy.
Antiemetics such as promethazine may mask the symptoms of an unrecognized disease and thereby interfere with diagnosis. Promethazine may cause marked drowsiness or impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. The use of alcohol or other central nervous system depressants such as sedatives/hypnotics (including barbiturates), narcotics, narcotic analgesics, general anesthetics, tricyclic antidepressants, and tranquilizers, may enhance impairment. Patients should be advised to report any involuntary muscle movements.
Avoid prolonged exposure to the sun.
Pregnancy
Meperidine should not be used in pregnant women prior to the labor period, unless in the judgment of the physician the potential benefits outweigh the possible risks, because safe use in pregnancy prior to labor has not been established relative to possible adverse effects on fetal development.
Teratogenic Effects
Promethazine is classified as Pregnancy Category C. Teratogenic effects have not been demonstrated in rat-feeding studies at doses of 6.25 and 12.5 mg/kg of promethazine hydrochloride. These doses are from approximately 2.1 to 4.2 times the maximum recommended total daily dose of promethazine for a 50 kg subject, depending upon the indication for which the drug is prescribed. Daily doses of 25 mg/kg intraperitoneally have been found to produce fetal mortality in rats.
Specific studies to test the action of the drug on parturition, lactation, and development of the animal neonate were not done, but a general preliminary study in rats indicated no effect on these parameters. Although antihistamines have been found to produce fetal mortality in rodents, the pharmacological effects of histamine in the rodent do not parallel those in man. There are no adequate and well controlled studies of promethazine in pregnant women. Promethazine hydrochloride tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS
The major hazards of meperidine, as with other narcotic analgesics, are respiratory depression and, to a lesser degree, circulatory depression; respiratory arrest, shock, and cardiac arrest have occurred.
The most frequently observed adverse reactions include light-headedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not experiencing severe pain. In such individuals, lower doses are advisable. Some adverse reactions in ambulatory patients may be alleviated if the patient lies down.
Other adverse reactions include
Central Nervous System: Euphoria, dysphoria, weakness, headache, agitation, tremor, uncoordinated muscle movements, transient hallucinations and disorientation, visual disturbances and, rarely, extrapyramidal reactions.
Gastrointestinal: Dry mouth, constipation, biliary-tract spasm.
Cardiovascular: Flushing of the face, tachycardia, bradycardia, palpitation, faintness, syncope.
Promethazine adverse reactions
Central Nervous System: Drowsiness is the most prominent CNS effect of this drug. Sedation, somnolence, blurred vision, dizziness; confusion, disorientation, and extrapyramidal symptoms such as oculogyric crisis, torticollis, and tongue protrusion; lassitude, tinnitus, incoordination, fatigue, euphoria, nervousness, diplopia, insomnia, tremors, convulsive seizures, excitation, catatonic-like states, hysteria. Hallucinations have also been reported.
Cardiovascular: Increased or decreased blood pressure, tachycardia, bradycardia, faintness.
Dermatologic: Dermatitis, photosensitivity, urticaria.
Hematologic: Leukopenia, thrombocytopenia, thrombocytopenic purpura, agranulocytosis.
Gastrointestinal: Dry mouth, nausea, vomiting, jaundice.
Respiratory: Asthma, nasal stuffiness, respiratory depression (potentially fatal) and apnea (potentially fatal).
Other: Angioneurotic edema. Neuroleptic malignant syndrome (potentially fatal) has also been reported.
Paradoxical Reactions: Hyperexcitability and abnormal movements have been reported in patients following a single administration of promethazine hydrochloride. Consideration should be given to the discontinuation of promethazine hydrochloride and to the use of other drugs if these reactions occur. Respiratory depression, nightmares, delirium, and agitated behavior have also been reported in some of these patients. Minor increases in blood pressure and occasional mild hypotension have been detected.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
To report SUSPECTED ADVERSE REACTIONS, contact ECI Pharmaceuticals, LLC at 954-486-8181 X-109
DRUG ABUSE AND DEPENDENCE
Tolerance and Addiction Liability
Drug Dependence
Meperidine can produce drug dependence of the morphine type and therefore has the potential for being abused. Psychic dependence, physical dependence, and tolerance may develop upon repeated administration of meperidine, and it should be prescribed and administered with the same degree of caution appropriate to the use of morphine. Like other narcotics, meperidine is subject to the provisions of the Federal narcotic laws for Schedule II drugs.
OVERDOSAGE
Symptoms
Serous overdose with meperidine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest, and death may occur.
Treatment
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patient airway and institution of assisted or controlled ventilation. The narcotic antagonist, naloxone hydrochloride, is a specific antidote against respiratory depression that may result from overdosage or unusual sensitivity to narcotics, including meperidine. Therefore, an appropriate dose of naloxone hydrochloride should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation.
An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
NOTE: In an individual physically dependent on narcotics, the administration of the usual dose of a narcotic antagonist will precipitate an acute withdrawal syndrome. The severity of this syndrome will depend on the degree of physical dependence and the dose of antagonist administered. The use of narcotic antagonists in such individuals should be avoided if possible. If a narcotic antagonist must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and only one-tenth to one-fifth the usual initial dose administered.
Attempted suicides with promethazine have resulted in deep sedation, coma, rarely convulsions and cardiorespiratory symptoms compatible with the depth of sedation present. Extrapyramidal reactions may be treated with anticholinergic antiparkinson agents, diphenhydramine, or barbiturates.
If severe hypotension occurs, levarterenol or phenylephrine may be indicated. Epinephrine is probably best avoided, since it has been suggested that promethazine overdosage could produce a partial alpha-adrenergic blockade.
A paradoxical reaction, characterized by hyperexcitability and nightmares, has been reported in children receiving large single doses of promethazine.
DOSAGE AND ADMINISTRATION
The usual dosage is one capsule every four to six hours as needed for the relief of pain.
HOW SUPPLIED
Meperidine HCl and Promethazine HCl capsules are red capsules imprinted "622", available in
Bottles of 30 capsules NDC: 51293-622-64
Bottles of 50 capsules NDC: 51293-622-67
Bottles of 100 capsules NDC: 51293-622-01
| MEPERIDINE HYDROCHLORIDE AND PROMETHAZINE HYDROCHLORIDE
meperidine hydrochloride and promethazine hydrochloride capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - ECI Pharmaceuticals LLC (962476029) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ECI Pharmaceuticals LLC | 962476029 | MANUFACTURE(51293-622) | |