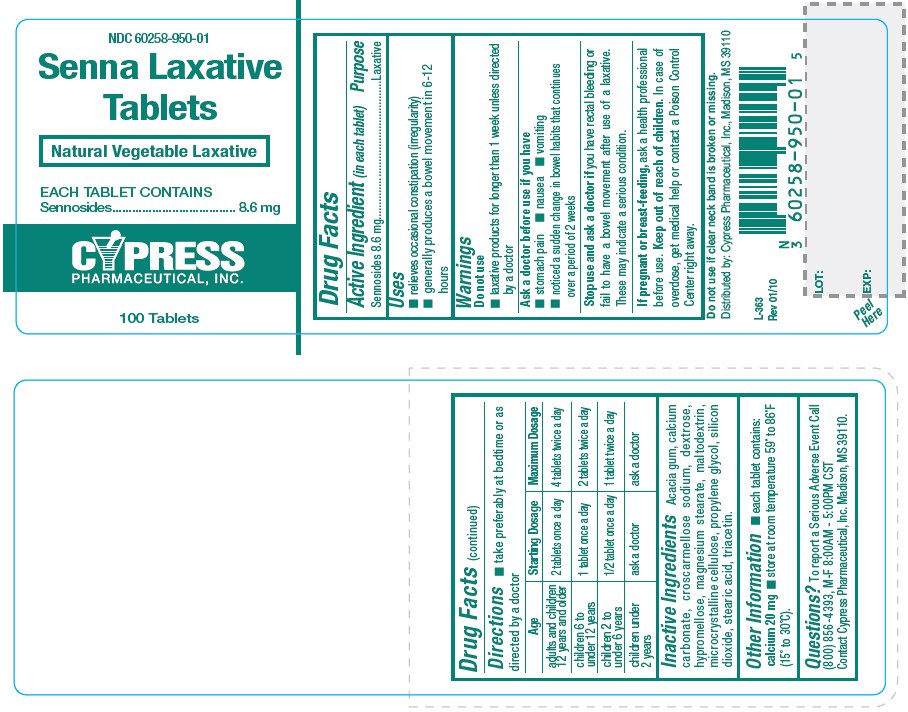
SENNA- sennosides tablet, film coated
Cypress Pharmaceutical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Senna
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6-12 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Directions
- take preferably at bedtime or as directed by a doctor
| Age | Starting Dosage | Maximum Dosage |
|---|---|---|
| adults and children 12 years and older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
Inactive Ingredients
Acacia gum, calcium carbonate, croscarmellose sodium, dextrose, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, propylene glycol, silicon dioxide, stearic acid, triacetin.
Other Information
- each tablet contains: calcium 20 mg
- store at room temperature 59° to 86°F (15° to 30°C).
| SENNA
sennosides tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Cypress Pharmaceutical, Inc. (790248942) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gemini Pharmaceuticals | 055942270 | MANUFACTURE(60258-950) | |
Revised: 9/2015
Document Id: 07042115-fe98-445e-ab3a-cbdd12315c78
Set id: db3e09ed-aa54-4506-98df-8d07b6a4a1a1
Version: 1
Effective Time: 20150921
Cypress Pharmaceutical, Inc.