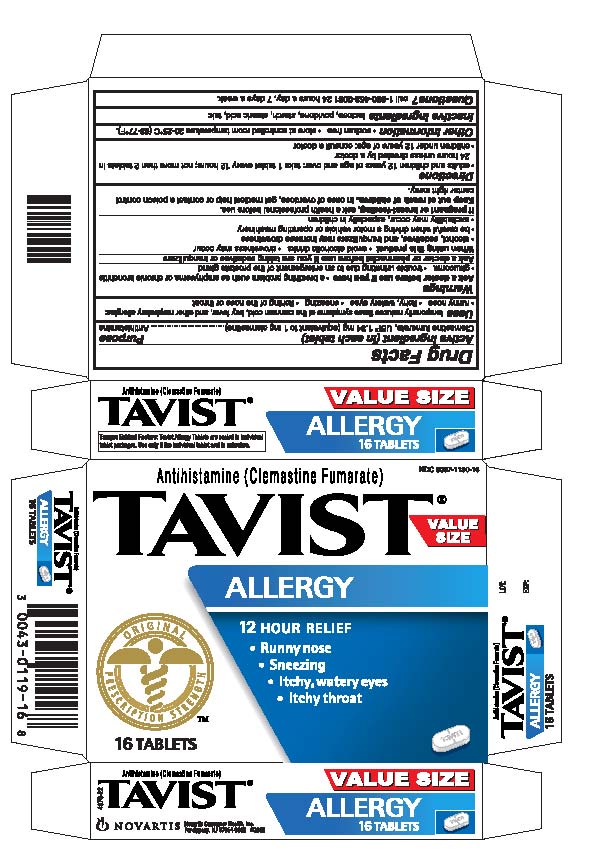
TAVIST ALLERGY- clemastine fumarate tablet
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
----------
Drug Facts
Uses
temporarily reduces these symptoms of the common cold, hay fever and other respiratory allergies:
• runny nose
• itchy, watery eyes
• sneezing
• itching of the nose or throat
Ask Doctor before use if you have
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- glaucoma
- •
- trouble urinating due to enlargement of the prostate gland
When using this product
- •
- avoid alcoholic drinks
- •
- drowsiness may occur
- •
- alcohol, sedative, and tranquillizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
- •
- excitability may occur, especially in children
Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- •
- adults and children 12 years of age and over: take 1 tablet every 12 hours; not more than 2 tablets in 24 hours unless direction by a doctor
- •
- children under 12 years of age: consult a doctor
| TAVIST
ALLERGY
clemastine fumarate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 1/2013
Document Id: e1bdf2e6-15bb-4de9-8fc7-25c903cff101
Set id: da09ea30-067f-45bc-9dc8-6fdd63db9ee1
Version: 3
Effective Time: 20130131
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC