Label: AVYCAZ- ceftazidime, avibactam powder, for solution
- NDC Code(s): 0456-2700-01, 0456-2700-10
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AVYCAZ® safely and effectively. See full prescribing information for AVYCAZ.
AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use
Initial U.S. Approval: 2015
RECENT MAJOR CHANGES
Indications and Usage (1.1, 1.2, 1.3) 1/2024 Dosage and Administration
(2.2)1/2024 Dosage of AVYCAZ in Pediatric Patients aged 2 years to less than 18 years with Estimated Glomerular Filtration Rate (eGFR) greater than 50 mL/min/1.73 m2 and in Pediatric Patients less than 2 years of age without Renal Impairment (2.2) Infection Age Range Dose Infusion Time/Frequency cIAI*, cUTI including Pyelonephritis, HABP/VABP 2 years to less than 18 years AVYCAZ 62.5 mg/kg to a maximum of 2.5 grams (ceftazidime 50 mg/kg and avibactam 12.5 mg/kg to a maximum dose of ceftazidime 2 grams and avibactam 0.5 grams) 2 hours/
Every 8 hours6 months to less than 2 years AVYCAZ 62.5 mg/kg (ceftazidime 50 mg/kg and avibactam 12.5 mg/kg) 3 months to less than 6 months AVYCAZ 50 mg/kg (ceftazidime 40 mg/kg and avibactam 10 mg/kg) Greater than 28 daysa to less than 3 months AVYCAZ 37.5 mg/kg
(ceftazidime 30 mg/kg and avibactam 7.5 mg/kg)Less than or equal to 28 daysb with GA 31 weeks and older AVYCAZ 25 mg/kg (ceftazidime 20 mg/kg and avibactam 5 mg/kg) INDICATIONS AND USAGE
AVYCAZ is a combination of ceftazidime, a cephalosporin, and avibactam, a beta-lactamase inhibitor, indicated for the treatment of the following infections caused by designated susceptible Gram-negative microorganisms in adult and pediatric patients (at least 31 weeks gestational age):
- Complicated Intra-abdominal Infections (cIAI), used in combination with metronidazole (1.1)
- Complicated Urinary Tract Infections (cUTI), including Pyelonephritis (1.2)
- Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP) (1.3)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of AVYCAZ and other antibacterial drugs, AVYCAZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.4)
DOSAGE AND ADMINISTRATION
Dosage of AVYCAZ in Adult Patients with Creatinine Clearance (CrCl) greater than 50 mL/min (2.1) Infection Dose Frequency Infusion Time cIAI*, cUTI including Pyelonephritis, and HABP/VABP AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam
0.5 grams)Every 8 hours 2 hours *Used in conjunction with metronidazole 0.5 g intravenously every 8 hours in adult cIAI patients *Used in conjunction with metronidazole 10 mg/kg intravenously every 8 hours in pediatric cIAI patients
aIncludes full-term infants with PNA > 28 days and pre-term infants with corrected age > 28 days. Corrected age is calculated by subtracting the number of weeks born before 40 weeks of gestation from the postnatal age.
bIncludes neonates PNA ≤ 28 days and pre-term infants with corrected age ≤ 28 days.
GA = gestational age and PNA = postnatal age.
- For treatment of cIAI, metronidazole should be given concurrently
- Recommended duration of treatment in adult and pediatric patients: (2.1, 2.2)
- cIAI: 5 to 14 days
- cUTI including pyelonephritis: 7 to 14 days
- HABP/VABP: 7 to 14 days
- cIAI: 5 to 14 days
- Dosage adjustment is required in adult patients with creatinine clearance (CrCl) less than 50 mL/min and in pediatric patients aged 2 years and older with renal impairment. There is insufficient information to recommend a dosing regimen for pediatric patients younger than 2 years of age with renal impairment. See Full Prescribing Information for additional information. (2.3)
- See Full Prescribing Information for instructions for constituting supplied dry powder and subsequent required dilution. (2.4)
- See Full Prescribing Information for drug compatibilities. (2.5)
DOSAGE FORMS AND STRENGTHS
AVYCAZ 2.5g (ceftazidime and avibactam) for injection is supplied as a sterile powder for constitution in single-dose vials containing ceftazidime 2 grams (equivalent to 2.635 grams of ceftazidime pentahydrate/sodium carbonate powder) and avibactam 0.5 grams (equivalent to 0.551 grams of avibactam sodium). (3)
CONTRAINDICATIONS
AVYCAZ is contraindicated in patients with known serious hypersensitivity to the components of AVYCAZ (ceftazidime and avibactam), avibactam-containing products or other members of the cephalosporin class. (4)
WARNINGS AND PRECAUTIONS
-
Decreased Clinical Response in Adult cIAI Patients with Baseline CrCl of 30 to Less Than or Equal to 50 mL/ min: Monitor CrCl at least daily in adult and pediatric patients with changing renal function and adjust the dosage of AVYCAZ accordingly. (5.1)
-
Hypersensitivity Reactions: Includes anaphylaxis and serious skin reactions. Cross sensitivity may occur in patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue AVYCAZ. (5.2)
-
Clostridioides difficile-associated Diarrhea (CDAD): CDAD has been reported with nearly all systemic antibacterial agents, including AVYCAZ. Evaluate if diarrhea occurs. (5.3)
- Central Nervous System Reactions: Seizures and other neurologic events may occur, especially in patients with renal impairment. Adjust dose in patients with renal impairment. (5.4)
ADVERSE REACTIONS
-
Adult Patients: The most common adverse reactions in cIAI (≥ 5%, when used with metronidazole) patients are diarrhea, nausea and vomiting. The most common adverse reactions (3%) in cUTI patients are diarrhea and nausea. The most common adverse reactions (≥ 5%) in HABP/VABP patients were diarrhea and vomiting. (6.1)
-
Pediatric Patients (aged 3 months to less than 18 years): The most common adverse reactions (≥ 3%) in pediatric patients aged 3 months and older were vomiting, diarrhea, rash, and infusion site phlebitis. (6.1)
- Pediatric Patients (less than 3 months of age): The most common adverse reactions (>3 %) in pediatric patients less than 3 months of age were vomiting and increased transaminases. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2024
- Complicated Intra-abdominal Infections (cIAI), used in combination with metronidazole (1.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Complicated Intra-abdominal Infections (cIAI)
1.2 Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
1.3 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
1.4 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adult Patients
2.2 Recommended Dosage in Pediatric Patients
2.3 Dosage Adjustments in Adult and Pediatric Patients (Aged 2 Years and Older) with Renal Impairment
2.4 Preparation of the AVYCAZ Solution for Administration
2.5 Drug Compatibility
2.6 Storage of Constituted Solutions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Clinical Response in Adult cIAI Patients with Baseline Creatinine Clearance of 30 to Less Than or Equal to 50 mL/min
5.2 Hypersensitivity Reactions
5.3 Clostridioides difficile-associated Diarrhea
5.4 Central Nervous System Reactions
5.5 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Probenecid
7.2 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Complicated Intra-abdominal Infections
14.2 Complicated Urinary Tract Infections, Including Pyelonephritis
14.3 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1
INDICATIONS AND USAGE
1.1 Complicated Intra-abdominal Infections (cIAI)
AVYCAZ (ceftazidime and avibactam) in combination with metronidazole, is indicated for the treatment of complicated intra-abdominal infections (cIAI) in adult and pediatric patients (at least 31 weeks gestational age) caused by the following susceptible gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter freundii complex, and Pseudomonas aeruginosa.
1.2 Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
AVYCAZ (ceftazidime and avibactam) is indicated for the treatment of complicated urinary tract infections (cUTI) including pyelonephritis in adult and pediatric patients (at least 31 weeks gestational age) caused by the following susceptible gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Citrobacter freundii complex, Proteus mirabilis, and Pseudomonas aeruginosa.
1.3 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
AVYCAZ (ceftazidime and avibactam) is indicated for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adult and pediatric patients (at least 31 weeks gestational age) caused by the following susceptible gram-negative microorganisms: Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
1.4 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of AVYCAZ and other antibacterial drugs, AVYCAZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2
DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adult Patients
The recommended dosage of AVYCAZ is 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours by intravenous (IV) infusion over 2 hours in patients 18 years of age and older with CrCl greater than 50 mL/min. For treatment of cIAI, metronidazole should be given concurrently. The guidelines for dosage of AVYCAZ in patients with creatinine clearance (CrCl) greater than 50 mL/min are listed in Table 1.
Table 1. Dosage of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) by Indication in Adult Patients (18 years of age and older) Infection Dose Frequency Infusion Time
(hours)Duration of Treatment Complicated Intra-abdominal
Infections (cIAI)*2.5 grams
Every 8 hours
2
cIAI: 5 to 14 days
cUTI: 7 to 14 days
HABP/VABP: 7 to 14 days
Complicated Urinary Tract
Infections including Pyelonephritis (cUTI)Hospital-acquired Bacterial
Pneumonia and Ventilator-associated
Bacterial Pneumonia (HABP/VABP)* Used in conjunction with metronidazole 0.5 g intravenously every 8 hours in adult cIAI patients [see Clinical Studies (14.1)]. 2.2 Recommended Dosage in Pediatric Patients
The recommended dosage of AVYCAZ in pediatric patients aged 2 years to less than 18 years and an estimated glomerular filtration rate (eGFR) greater than 50 mL/min/1.73 m2 and in pediatric patients less than 2 years of age without renal impairment is described in Table 2. AVYCAZ is administered every 8 hours by intravenous infusion over 2 hours. For treatment of cIAI, metronidazole should be given concurrently.
Table 2. Dosage of AVYCAZ (ceftazidime and avibactam) in Pediatric Patients Infection Age Range Dose Frequency Infusion Time (hours) Duration of treatment cIAI*, cUTI including Pyelonephritis, and HABP/VABP 2 years to less
than 18 yearsaAVYCAZ 62.5 mg/kg to a maximum of 2.5 grams (ceftazidime 50 mg/kg and avibactam 12.5 mg/kg to a maximum dose of ceftazidime 2 grams and avibactam 0.5 grams)
Every 8 hours 2
cIAI: 5 to 14 days
cUTI: 7 to 14 days HABP/VABP: 7 to 14 days
6 months to less than 2 years AVYCAZ 62.5 mg/kg (ceftazidime 50 mg/kg and avibactam 12.5 mg/kg) 3 months to less than 6 months AVYCAZ 50 mg/kg (ceftazidime 40 mg/kg and avibactam 10 mg/kg) Greater than 28 daysb to less than 3 months AVYCAZ 37.5 mg/kg (ceftazidime 30 mg/kg and avibactam 7.5 mg/kg) Less than or equal to 28 daysc with GA 31 weeks and older AVYCAZ 25 mg/kg (ceftazidime 20 mg/kg and avibactam 5 mg/kg) *AVYCAZ was used in conjunction with metronidazole 10 mg/kg intravenously every 8 hours in pediatric cIAI patients [see Clinical Studies (14.1)]
a For pediatric patients (aged 2 years and older) with eGFR less than or equal to 50 mL/min/1.73m2, dosage adjustments are recommended [see Dosage and Administration (2.3)].
b Includes full-term infants with PNA > 28 days and pre-term infants with corrected age > 28 days. Corrected age is calculated by subtracting the number of weeks born before 40 weeks of gestation from the postnatal age.
c Includes neonates PNA ≤ 28 days and pre-term infants with corrected age ≤ 28 days.
GA = gestational age and PNA = postnatal age.
2.3 Dosage Adjustments in Adult and Pediatric Patients (Aged 2 Years and Older) with Renal Impairment
The recommended AVYCAZ dosage in adult and pediatric patients aged 2 years and older with varying degrees of renal function is presented in Table 3 and Table 4, respectively. For patients with changing renal function, monitor CrCl in adults or eGFR in pediatric patients at least daily and adjust the dosage of AVYCAZ accordingly [see Warnings and Precautions (5.1), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. There is insufficient information to recommend a dosing regimen for pediatric patients less than 2 years of age with renal impairment.
Adult Patients
Table 3. Dosage of AVYCAZ in Adult Patients with Renal Impairment Estimated Creatinine Clearance (mL/minute)a Dose for AVYCAZ (ceftazidime and avibactam)b Frequency 31 to 50 AVYCAZ 1.25 grams (ceftazidime 1 gram and avibactam 0.25 grams) intravenously Every 8 hours 16 to 30 AVYCAZ 0.94 grams (ceftazidime 0.75 grams and avibactam 0.19 grams) intravenously Every 12 hours 6 to 15c AVYCAZ 0.94 grams (ceftazidime 0.75 grams and avibactam 0.19 grams) intravenously Every 24 hours Less than or equal to 5c AVYCAZ 0.94 grams (ceftazidime 0.75 grams and avibactam 0.19 grams) intravenously Every 48 hours a As calculated using the Cockcroft-Gault formula
b All doses of AVYCAZ are administered over 2 hours
c Both ceftazidime and avibactam are hemodialyzable; thus, administer AVYCAZ after hemodialysis on hemodialysis daysPediatric Patients
Table 4. Dosage of AVYCAZ in Pediatric Patients Aged 2 years and older with Renal Impairmenta Estimated eGFRb (mL/min/1.73m2) Dose for AVYCAZ (ceftazidime and avibactam)c Frequency 31 to 50 AVYCAZ 31.25 mg/kg to a maximum of 1.25 grams (ceftazidime 25 mg/kg and avibactam 6.25 mg/kg to a maximum dose of ceftazidime 1 gram and avibactam 0.25 grams) Every 8 hours 16 to 30 AVYCAZ 23.75 mg/kg to a maximum of 0.94 grams (ceftazidime 19 mg/kg and avibactam 4.75 mg/kg to a maximum dose of ceftazidime 0.75 grams and avibactam 0.19 grams) Every 12 hours 6 to 15 AVYCAZ 23.75 mg/kg to a maximum of 0.94 grams (ceftazidime 19 mg/kg and avibactam 4.75 mg/kg to a maximum dose of ceftazidime 0.75 grams and avibactam 0.19 grams) Every 24 hours Less than or equal to 5d AVYCAZ 23.75 mg/kg to a maximum of 0.94 grams (ceftazidime 19 mg/kg and avibactam 4.75 mg/kg to a maximum dose of ceftazidime 0.75 grams and avibactam 0.19 grams) Every 48 hours a Dosing was derived based on the population PK modeling, which assumed similar proportional effects of renal impairment in adults and pediatric
patients aged 2 years and older [see Clinical Pharmacology (12.3)]
b As calculated using the Schwartz bedside formula
c All doses of AVYCAZ are administered over 2 hours
d Both ceftazidime and avibactam are hemodialyzable; thus, administer AVYCAZ after hemodialysis on hemodialysis days2.4 Preparation of the AVYCAZ Solution for Administration
AVYCAZ is supplied as a dry powder, which must be constituted and subsequently diluted, using aseptic technique prior to intravenous infusion.
a) Constitute the powder in the AVYCAZ vial with 10 mL of one of the following solutions:
- sterile water for injection, USP
- 0.9% of sodium chloride injection, USP (normal saline)
- 5% of dextrose injection, USP
- all combinations of dextrose injection and sodium chloride injection, USP, containing up to 2.5% dextrose, USP, and 0.45% sodium chloride, USP, or
- lactated Ringer’s injection, USP
b) Mix gently and ensure that the contents are dissolved completely. The constituted AVYCAZ solution will have an approximate ceftazidime concentration of 167 mg/mL and an approximate avibactam concentration of 42 mg/mL. The final volume is approximately 12 mL. The constituted solution is not for direct injection. The constituted solution must be diluted before intravenous infusion.
c) Prepare the required dose for intravenous infusion by withdrawing the appropriate volume determined from Table 5 from the constituted vial. To prepare doses for pediatric patients weighing less than 40 kg, follow the constitution instruction above to yield a solution with a final AVYCAZ concentration of approximately 209 mg/mL (ceftazidime concentration of 167 mg/mL and an avibactam concentration of 42 mg/mL). Use these concentrations to calculate the volume of AVYCAZ required to prepare the prescribed dose.
Table 5. Preparation of AVYCAZ Doses for Adult and Pediatric Patients (Weighing 40 kg or More) AVYCAZ (ceftazidime and avibactam) Dose Volume to Withdraw from Constituted Vial for Further Dilution to
50 to 250a mL2.5 grams (2 grams and 0.5 grams) 12 mL (entire contents) 1.25 grams (1 gram and 0.25 grams) 6 mL 0.94 grams (0.75 grams and 0.19 grams) 4.5 mL a. Dilution to 250 mL should only be used for the 2.5 gram dose d) Before infusion, dilute the withdrawn volume of the constituted AVYCAZ solution further with the same diluent used for constitution of the powder (except sterile water for injection), to achieve a ceftazidime concentration of 8 to 40 mg/mL and an avibactam concentration of 2 to 10 mg/mL in an infusion bag. If sterile water for injection was used for constitution, use any of the other appropriate constitution diluents for dilution.
e) Mix gently and ensure that the contents are dissolved completely. Visually inspect the diluted AVYCAZ solution (for administration) for particulate matter and discoloration prior to administration (the color of the AVYCAZ infusion solution for administration ranges from clear to light yellow).
f) Use the diluted AVYCAZ solution in the infusion bags within 12 hours when stored at room temperature.
g) The diluted AVYCAZ solution in the infusion bags may be stored under refrigeration at 2 to 8°C (36 to 46°F) up to 24 hours following dilution and used within 12 hours of subsequent storage at room temperature.
2.5 Drug Compatibility
The AVYCAZ solution for administration at the range of diluted concentrations of ceftazidime 8 mg/mL and avibactam 2 mg/mL to ceftazidime 40 mg/mL and avibactam 10 mg/mL is compatible with the more commonly used intravenous infusion fluids in infusion bags (including Baxter® Mini-Bag Plus™) such as:
- 0.9% sodium chloride injection, USP
- 5% dextrose injection, USP
- all combinations of dextrose injection and sodium chloride injection, USP, containing up to 2.5% dextrose, USP, and 0.45% sodium chloride, USP
- lactated ringer's injection, USP, and
- Baxter® Mini-Bag Plus™ containing 0.9% sodium chloride injection or 5% dextrose injection
Intravenous Line Compatibility
Simulated Y-site compatibility of AVYCAZ admixed with other drug products in a 1:1 volume ratio at room temperature was evaluated by visual inspection, and measurement of turbidity and particulate matter at 0, 1 and 4 hours after mixing. Ceftazidime and avibactam were tested at concentrations of 20 mg/mL and 5 mg/mL, respectively, which can be obtained by dilution of constituted AVYCAZ solution in a 100 mL intravenous infusion bag. The highest recommended concentration (40 mg/mL of ceftazidime and 10 mg/mL of avibactam) was not tested in this study and should not be used during co-administration of AVYCAZ with other drugs through the same intravenous line. Compatible drugs with the corresponding compatible diluent (i.e., 0.9% Sodium Chloride Injection, 5 % Dextrose Injection or Lactated Ringer’s Injection) are listed in Tables 6, 7, 8 and 9 below. Any drug products not listed in the tables below should not be co-administered with AVYCAZ through the same intravenous line (or cannula).
Table 6. Compatible Drugs for use with 0.9% Sodium Chloride, 5% Dextrose or Lactated Ringer’s Injection as Diluents Daptomycin Dexmedetomidine Injection Dopamine Hydrochloride Injection Furosemide Injection Gentamicin Injection Imipenem and Cilastatin for Injection Magnesium Sulfate Injection Norepinephrine Bitartrate Injection Phenylephrine Hydrochloride Injection Vasopressin Injection Vecuronium Bromide Metronidazole Injection Aztreonam Injection or Aztreonam for Injection Colistimethate for Injection Amikacin Sulfate Injection Azithromycin for Injection Ceftaroline fosamil for Injection Levofloxacin Table 7. Compatible Drugs for use with 0.9% Sodium Chloride or 5% Dextrose Injection as Diluents Ertapenem Sodium Potassium Phosphates Injection Table 8. Compatible Drugs for use with 5% Dextrose or Lactated Ringer’s Injection as Diluents Heparin Sodium Injection Linezolid Injection Tobramycin Injection or Tobramycin for Injection Table 9. Compatible Drugs for use with One Compatible Diluent only Meropenem for Injection (0.9% Sodium Chloride Injection diluent only) Sodium Bicarbonate Injection (5% Dextrose Injection diluent only) Tedizolid Phosphate for Injection (5% Dextrose Injection diluent only) Potassium Chloride in Water for Injection (40 mEq/100 mL) (Lactated Ringer’s Injection diluent only) 2.6 Storage of Constituted Solutions
Upon constitution with appropriate diluent, the constituted AVYCAZ solution may be held for no longer than 30 minutes prior to transfer and dilution in a suitable infusion bag.
Following dilution of the constituted solutions with the appropriate diluents, AVYCAZ solutions in the infusion bags are stable for 12 hours when stored at room temperature.
Following dilution of the constituted solutions with the appropriate diluents, AVYCAZ solutions in the infusion bags may also be refrigerated at 2 to 8°C (36 to 46°F) for up to 24 hours; and then should be used within 12 hours of subsequent storage at room temperature.
- sterile water for injection, USP
-
3
DOSAGE FORMS AND STRENGTHS
AVYCAZ 2.5 grams (ceftazidime and avibactam) for injection is supplied as a white to yellow sterile powder for constitution in a single-dose, sterile, clear glass vial containing ceftazidime 2 grams (equivalent to 2.635 grams of ceftazidime pentahydrate/sodium carbonate powder) and avibactam 0.5 grams (equivalent to 0.551 grams of avibactam sodium).
-
4
CONTRAINDICATIONS
AVYCAZ is contraindicated in patients with known serious hypersensitivity to the components of AVYCAZ (ceftazidime and avibactam), avibactam containing products, or other members of the cephalosporin class [see Warnings and Precautions (5.2)].
-
5
WARNINGS AND PRECAUTIONS
5.1 Decreased Clinical Response in Adult cIAI Patients with Baseline Creatinine Clearance of 30 to Less Than or Equal to 50 mL/min
In a Phase 3 cIAI trial in adult patients, clinical cure rates were lower in a subgroup of patients with baseline CrCl of 30 to less than or equal to 50 mL/min compared to those with CrCl greater than 50 mL/min (Table 10). The reduction in clinical cure rates was more marked in patients treated with AVYCAZ plus metronidazole compared to meropenem-treated patients. Within this subgroup, patients treated with AVYCAZ received a 33% lower daily dose than is currently recommended for patients with CrCl 30 to less than or equal to 50 mL/min.
The decreased clinical response was not observed for patients with moderate renal impairment at baseline (CrCl of 30 to less than or equal to 50 mL/min) in the Phase 3 cUTI trials or the Phase 3 HABP/VABP trial.
Monitor CrCl at least daily in adult and pediatric patients with changing renal function and adjust the dosage of AVYCAZ accordingly [see Dosage and Administration (2.2, 2.3), and Adverse Reactions (6.1)].
Table 10. Clinical Cure Rate at Test of Cure in a Phase 3 cIAI Trial, by Baseline Renal Function – mMITT Populationa AVYCAZ + Metronidazole
% (n/N)Meropenem
% (n/N)Normal function / mild impairment
(CrCl greater than 50 mL/min)85% (322/379) 86% (321/373) Moderate impairment
(CrCl 30 to less than or equal to 50 mL/min)45% (14/31) 74% (26/35) a Microbiological modified intent-to-treat (mMITT) population included patients who had at least one bacterial pathogen at baseline and received at least one dose of study drug. 5.2 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam antibacterial drugs. Before therapy with AVYCAZ is instituted, careful inquiry about previous hypersensitivity reactions to other cephalosporins, penicillins, or carbapenems should be made. Exercise caution if this product is to be given to a penicillin or other beta-lactam-allergic patient because cross sensitivity among beta-lactam antibacterial drugs has been established. Discontinue the drug if an allergic reaction to AVYCAZ occurs.
5.3 Clostridioides difficile-associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial drugs, including AVYCAZ, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial drugs alters the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial drugs.
If CDAD is suspected or confirmed, antibacterial drugs not directed against C. difficile may need to be discontinued. Manage fluid and electrolyte levels as appropriate, supplement protein intake, monitor antibacterial treatment of C. difficile, and institute surgical evaluation as clinically indicated.
5.4 Central Nervous System Reactions
Seizures, nonconvulsive status epilepticus (NCSE), encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia have been reported in patients treated with ceftazidime, particularly in the setting of renal impairment. Adjust dosing based on creatinine clearance [see Dosage and Administration (2.2)].
5.5 Development of Drug-Resistant Bacteria
Prescribing AVYCAZ in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria [see Indications and Usage (1.4)].
-
6
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in the Warnings and Precautions section:
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
-
Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.3)]
- Central Nervous System Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Patients
AVYCAZ was evaluated in six active-controlled clinical trials in patients with cIAI, cUTI, including pyelonephritis, or HABP/VABP. These trials included two Phase 2 trials, one in cIAI and one in cUTI, as well as four Phase 3 trials, one in cIAI, one in cUTI (Trial 1), one in cIAI or cUTI due to ceftazidime non-susceptible pathogens (Trial 2), and one in HABP/VABP. Data from cUTI Trial 1 served as the primary dataset for AVYCAZ safety findings in cUTI as there was a single comparator. cUTI Trial 2 had an open-label design as well as multiple comparator regimens which prevented pooling but provided supportive information. The six clinical trials included a total of 1809 adult patients treated with AVYCAZ and 1809 patients treated with comparators.
Complicated Intra-abdominal Infections
The Phase 3 cIAI trial included 529 adult patients treated with AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes every 8 hours plus 0.5 grams metronidazole administered intravenously over 60 minutes every 8 hours and 529 patients treated with meropenem. The median age of patients treated with AVYCAZ was 50 years (range 18 to 90 years) and 22.5% of patients were 65 years of age or older. Patients were predominantly male (62%) and Caucasian (76.6%).
Treatment discontinuation due to an adverse reaction occurred in 2.6% (14/529) of patients receiving AVYCAZ plus metronidazole and 1.3% (7/529) of patients receiving meropenem.
Adverse reactions occurring at 5% or greater in patients receiving AVYCAZ plus metronidazole were diarrhea, nausea, and vomiting.
Table 11 lists adverse reactions occurring in 1% or more of patients receiving AVYCAZ plus metronidazole and with incidences greater than the comparator in the Phase 3 cIAI clinical trial.
Table 11. Incidence of Selected Adverse Reactions Occurring in 1% or more of Adult Patients (18 years of age and older) Receiving AVYCAZ in the Phase 3 cIAI Trial Adverse Reactions AVYCAZ plus metronidazolea
(N=529)Meropenemb
(N=529)Nervous system disorders Headache 3% 2% Dizziness 2% 1% Gastrointestinal disorders Diarrhea 8% 3% Nausea 7% 5% Vomiting 5% 2% Abdominal Pain 1% 1% a 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV over 120 minutes every 8 hours (with metronidazole 0.5 grams IV every 8 hours)
b 1 gram IV over 30 minutes every 8 hoursIncreased Mortality
In the Phase 3 cIAI trial, death occurred in 2.5% (13/529) of patients who received AVYCAZ plus metronidazole and in 1.5% (8/529) of patients who received meropenem. Among a subgroup of patients with baseline CrCl 30 to less than or equal to 50 mL/min, death occurred in 19.5% (8/41) of patients who received AVYCAZ plus metronidazole and in 7.0% (3/43) of patients who received meropenem. Within this subgroup, patients treated with AVYCAZ received a 33% lower daily dose than is currently recommended for patients with CrCl 30 to less than or equal to 50 mL/min [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)]. In patients with normal renal function or mild renal impairment (baseline CrCl greater than 50 mL/min), death occurred in 1.0% (5/485) of patients who received AVYCAZ plus metronidazole and in 1.0% (5/484) of patients who received meropenem. The causes of death varied and contributing factors included progression of underlying infection, baseline pathogens isolated that were unlikely to respond to the study drug, and delayed surgical intervention.
Complicated Urinary Tract Infections, Including Pyelonephritis
The Phase 3 cUTI Trial 1 included 511 adult patients treated with AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes every 8 hours and 509 patients treated with doripenem; in some patients parenteral therapy was followed by a switch to an oral antimicrobial agent [see Clinical Studies (14.2)]. Median age of patients treated with AVYCAZ was 54 years (range 18 to 89 years) and 30.7% of patients were 65 years of age or older. Patients were predominantly female (68.3%) and Caucasian (82.4%). Patients with CrCl less than 30 mL/min were excluded.
There were no deaths in Trial 1. Treatment discontinuation due to adverse reactions occurred in 1.4% (7/511) of patients receiving AVYCAZ and 1.2% (6/509) of patients receiving doripenem.
The most common adverse reactions occurring in 3% of cUTI patients treated with AVYCAZ were nausea and diarrhea.
Table 12 lists adverse reactions occurring in 1% or more of patients receiving AVYCAZ and with incidences greater than the comparator in Trial 1.
Table 12. Incidence of Selected Adverse Drug Reactions Occurring in 1% or more of Adult Patients (18 years of age and older) Receiving AVYCAZ in the Phase 3 cUTI Trial 1 Adverse Reactions AVYCAZa
(N=511)
Doripenemb
(N=509)
Gastrointestinal disorders Nausea 3% 2% Diarrhea 3% 1% Constipation 2% 1% Upper abdominal pain 1% < 1% a 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV over 120 minutes every 8 hours
b 0.5 grams IV over 60 minutes every 8 hoursHospital-acquired Bacterial Pneumonia/Ventilator-associated Bacterial Pneumonia
The Phase 3 HABP/VABP trial included 436 adult patients treated with AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes and 434 patients treated with meropenem. The median age of patients treated with AVYCAZ was 66 years (range 18 to 89 years) and 54.1% of patients were 65 years of age or older. Patients were predominantly male (74.5%) and Asian (56.2%).
Death occurred in 9.6% (42/ 436) of patients who received AVYCAZ and in 8.3% (36/434) of patients who received meropenem. Treatment discontinuation due to an adverse reaction occurred in 3.7% (16/436) of patients receiving AVYCAZ and 3% (13/434) of patients receiving meropenem.
Adverse reactions occurring at 5% or greater in patients receiving AVYCAZ were diarrhea and vomiting.
Table 13 lists selected adverse reactions occurring in 1% or more of patients receiving AVYCAZ and with incidences greater than the comparator in the Phase 3 HABP/VABP clinical trial.
Table 13. Incidence of Selected Adverse Drug Reactions Occurring in 1% or more of Adult Patients (18 years of age and older) Receiving AVYCAZ in the Phase 3 HABP/VABP Trial Adverse Reactions AVYCAZa
(N=436)Meropenemb
(N=434)Gastrointestinal disorders Nausea 3% 2% Skin and subcutaneous tissue disorders Pruritus 2% 1% a 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV over 120 minutes every 8 hours
b 1 gram IV over 30 minutes every 8 hoursOther Adverse Reactions of AVYCAZ and Ceftazidime in Adults
Direct Coombs’ Test Seroconversion with AVYCAZ
In the Phase 3 trials, seroconversion from a negative to a positive direct Coombs’ test result among patients with an initial negative Coombs’ test and at least one follow up test occurred in 3% (cUTI), 12.9% (cIAI), and 21.4% (HABP/VABP) of patients receiving AVYCAZ and 0.9% (cUTI), 3% (cIAI) and 7% (HABP/VABP) of patients receiving a carbapenem comparator.
Less Common Adverse Reactions with AVYCAZ
The following selected adverse reactions were reported in AVYCAZ-treated patients at a rate of less than 1% in the Phase 3 trials and are not described elsewhere in the labeling.
Blood and lymphatic disorders – Thrombocytopenia, Thrombocytosis, Leukopenia
General disorders and administration site conditions – Injection site phlebitis
Infections and infestations – Candidiasis
Investigations – Increased aspartate aminotransferase, Increased alanine aminotransferase, Increased gamma-glutamyl transferase
Metabolism and nutrition disorders – Hypokalemia
Nervous system disorders – Dysgeusia
Renal and urinary disorders – Acute kidney injury, Renal impairment, Nephrolithiasis
Skin and subcutaneous tissue disorders – Rash, Rash maculo-papular, Urticaria
Psychiatric disorders – AnxietyAdverse Reactions with Ceftazidime
Additionally, adverse reactions reported with ceftazidime alone that were not reported in AVYCAZ-treated patients in the Phase 3 trials are listed below:
Blood and lymphatic disorders – Agranulocytosis, Hemolytic anemia, Lymphocytosis, Neutropenia, Eosinophilia
General disorders and administration site conditions – Infusion site inflammation, Injection site hematoma, Injection site thrombosis
Hepatobiliary disorders – Jaundice
Investigations – Increased blood lactate dehydrogenase, Prolonged prothrombin time
Nervous system disorders – Paresthesia, seizures, encephalopathy, coma, asterixis, neuromuscular excitability, myoclonia
Renal and urinary disorders – Tubulointerstitial nephritis
Reproductive and breast disorders – Vaginal inflammation
Hypersensitivity Reactions– Anaphylaxis, Angioedema, Erythema multiforme, Stevens-Johnson syndrome, Toxic epidermal necrolysisClinical Trials Experience in Pediatric Patients
Pediatric Patients Aged 3 months to less than 18 years
AVYCAZ was evaluated in 128 pediatric patients aged 3 months to < 18 years in two single-blind, randomized, active-controlled clinical trials, one in patients with cUTI and the other in patients with cIAI. Safety data from the two studies were pooled. The AVYCAZ dosing regimen was the same in both of these trials [see Dosage and Administration (2.2)] with a mean treatment duration of 6 days, and a maximum of 14 days. The regimen was selected to result in pediatric drug exposure comparable to that of adults, and in the cIAI trial, metronidazole was administered concurrently with AVYCAZ. Patients were randomized 3:1 to receive AVYCAZ or comparator, which was meropenem or cefepime in the cIAI and cUTI trials, respectively. The median age of patients treated with AVYCAZ was 8.6 years, and in the comparator group 7.4 years. The majority of patients treated with AVYCAZ were female (57%) and Caucasian (80%). An open-label single-dose pharmacokinetic (PK) and safety trial was conducted in pediatric patients with HABP/VABP and enrolled four patients aged 11.6 months to 9.4 years [see Clinical Pharmacology 12.3].
There were no deaths reported in the trials of cUTI, cIAI, and HABP/VABP in pediatric patients aged 3 months and older. Treatment discontinuation due to adverse reactions in the pediatric cUTI and cIAI trials occurred in 2.3% (3/128) of patients receiving AVYCAZ and 0/50 of patients receiving comparator drugs.
The most common adverse reactions occurring in greater than 3% of pediatric patients aged 3 months to < 18 years treated with AVYCAZ were vomiting, diarrhea, rash, and infusion site phlebitis.
Pediatric Patients less than 3 months of Age
AVYCAZ was also evaluated in a trial enrolling 46 pediatric patients less than three months of age as follows: infants > 28 days to < 3 months (N=17), term neonates from birth to 28 days, (N=13), pre-term neonates from birth (gestational age ≥ 31 weeks) to 28 days (N=16). The median age of patients treated with AVYCAZ was 24 days. In this single-arm trial, 25 patients with a suspected or confirmed bacterial infection received a single-dose of AVYCAZ and 21 patients with suspected or confirmed serious gram-negative infections received multiple doses of AVYCAZ [see Dosage and Administration (2.2)]. The demographics of patients treated with AVYCAZ were female (54%), male (46%); racial groups of White (78%), Asian (11%), Black or African American (9%); ethnicities of Not Hispanic or Latino (91.3%); Hispanic or Latino (4.3%). In patients treated with multiple doses of AVYCAZ [see Dosage and Administration (2.2)], the mean treatment duration was 6 days and maximum treatment duration was 12 days.
There was one death reported in the trial for pediatric patients less than 3 months of age. There were no treatment discontinuations due to adverse reactions. The most common adverse reactions occurring in greater than 3% of pediatric patients less than 3 months of age were vomiting and increased transaminases.
The safety profile of AVYCAZ in pediatric patients was similar to adults with cIAI, cUTI, and HABP/VABP treated with AVYCAZ.
6.2 Postmarketing Experience
The following adverse reactions and altered laboratory tests have been identified during post approval use of ceftazidime (a component of AVYCAZ), or other cephalosporin-class antibacterial drugs. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Colitis, toxic nephropathy, hepatic dysfunction including cholestasis, hemorrhage, pancytopenia, aplastic anemia, prolonged prothrombin time, false-positive test for urinary glucose.
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
-
7
DRUG INTERACTIONS
7.1 Probenecid
In vitro, avibactam is a substrate of OAT1 and OAT3 transporters which might contribute to the active uptake from the blood compartment, and thereby its excretion. As a potent OAT inhibitor, probenecid inhibits OAT uptake of avibactam by 56% to 70% in vitro and, therefore, has the potential to decrease the elimination of avibactam when co-administered. Because a clinical interaction study of AVYCAZ or avibactam alone with probenecid has not been conducted, co-administration of AVYCAZ with probenecid is not recommended [see Clinical Pharmacology (12.3)].
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of AVYCAZ, ceftazidime, or avibactam in pregnant women. Neither ceftazidime nor avibactam were teratogenic in rats at doses 40 and 9 times the recommended human clinical dose. In the rabbit, at twice the exposure as seen at the human clinical dose, there were no effects on embryofetal development with avibactam.
The background risk of major birth defects and miscarriage for the indicated population is unknown. The background risk of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies within the general population. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
Data
Animal Data
Ceftazidime
Reproduction studies have been performed in mice and rats at doses up to 40 times the human dose and showed no evidence of harm to the fetus due to ceftazidime.
Avibactam
Avibactam was not teratogenic in rats or rabbits. In the rat, intravenous studies with 0, 250, 500 and 1000 mg/kg/day avibactam during gestation days 6-17 showed no embryofetal toxicity at doses up to 1000 mg/kg/day, approximately 9 times the human dose based on exposure (AUC). In a rat pre- and post-natal study at up to 825 mg/kg/day intravenously (11 times the human exposure based on AUC), there were no effects on pup growth and viability. A dose-related increase in the incidence of renal pelvic and ureter dilatation was observed in female weaning pups that was not associated with pathological changes to renal parenchyma or renal function, with renal pelvic dilatation persisting after female weaning pups became adults.
Rabbits administered intravenous avibactam on gestation days 6-19 at 0, 100, 300 and 1000 mg/kg/day showed no effects on embryofetal development at a dose of 100 mg/kg, twice the human exposure (AUC). At higher doses, increased post-implantation loss, lower mean fetal weights, delayed ossification of several bones and other anomalies were observed.
8.2 Lactation
Risk Summary
Ceftazidime is excreted in human milk in low concentrations. It is not known whether avibactam is excreted into human milk, although avibactam was shown to be excreted in the milk of rats. No information is available on the effects of ceftazidime and avibactam on the breast-fed child or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AVYCAZ and any potential adverse effects on the breastfed child from AVYCAZ or from the underlying maternal conditions.
Data
In a rat pre- and post-natal study at doses up to 825 mg/kg/day intravenously (11 times the human exposure based on AUC), the exposure to avibactam was minimal in the pups in comparison to the dams. Exposure to avibactam was observed in both pups and milk on PND 7.
8.4 Pediatric Use
The safety and effectiveness of AVYCAZ in the treatment of cUTI, cIAI, and HABP/VABP have been established in pediatric patients at least 31 weeks gestational age and older. Use of AVYCAZ is supported by evidence from adequate and well-controlled studies of AVYCAZ in adults with cUTI, cIAI, and HABP/VABP and additional pharmacokinetic and safety data from pediatric trials [see Clinical Pharmacology (12.3), Clinical Studies (14.1 and 14.2)].
The safety profile of AVYCAZ in pediatric patients was similar to adults with cIAI, cUTI, and HABP/VABP treated with AVYCAZ [see Adverse Reactions (6.1)].
The safety and effectiveness of AVYCAZ in the treatment of cUTI, cIAI, and HABP/VABP have not been established in pediatric patients less than 31 weeks gestational age.
8.5 Geriatric Use
Of the 1809 patients treated with AVYCAZ in the Phase 2 and Phase 3 clinical trials 621 (34.5%) were 65 years of age and older, including 302 (16.7 %) patients 75 years of age and older.
In the pooled Phase 2 and Phase 3 cIAI AVYCAZ clinical trials, 20% (126/630) of patients treated with AVYCAZ were 65 years of age and older, including 49 (7.8%) patients 75 years of age and older. The incidence of adverse reactions in both treatment groups was higher in older patients (≥ 65 years of age) and similar in both treatment groups; clinical cure rates for patients 65 years of age or older were 73.0% (73/100) in the AVYCAZ plus metronidazole arm and 78.6% (77/98) in the meropenem arm.
In the Phase 3 cUTI trial, 30.7% (157/511) of patients treated with AVYCAZ were 65 years of age or older, including 78 (15.3%) patients 75 years of age or older. The incidence of adverse reactions in both treatment groups was lower in older patients (≥ 65 years of age) and similar between treatment groups. Among patients 65 years of age or older in the Phase 3 cUTI trial, 66.1% (82/124) of patients treated with AVYCAZ had symptomatic resolution at Day 5 compared with 56.6% (77/136) of patients treated with doripenem. The combined response (microbiological cure and symptomatic response) observed at the test-of-cure (TOC) visit for patients 65 years of age or older were 58.1% (72/124) in the AVYCAZ arm and 58.8% (80/136) in the doripenem arm.
In the Phase 3 HABP/VABP trial, 54.1% (236/436) of patients treated with AVYCAZ were 65 years of age or older, including 129 (29.6%) patients 75 years of age or older. The incidence of adverse reactions in patients ≥ 65 years of age was similar to patients < 65 years of age. The 28-day all-cause mortality was similar between treatment groups for patients 65 years of age or older (12.7% [29/229] for patients in the AVYCAZ arm and 11.3% [26/230] for patients in the meropenem arm).
Ceftazidime and avibactam are known to be substantially excreted by the kidney; therefore, the risk of adverse reactions to ceftazidime and avibactam may be greater in patients with decreased renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. Healthy elderly subjects had 17% greater exposure relative to healthy young subjects when administered the same single dose of avibactam, which may have been related to decreased renal function in the elderly subjects. Dosage adjustment for elderly patients should be based on renal function [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Dosage adjustment is required in adult patients with moderately or severely impaired renal function (CrCl 50 mL/min or less). For patients with changing renal function, CrCl should be monitored at least daily, particularly early in treatment, and dosage of AVYCAZ adjusted accordingly. Both ceftazidime and avibactam are hemodialyzable; thus, AVYCAZ should be administered after hemodialysis on hemodialysis days [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
Dosage adjustment is also required in pediatric patients with renal impairment from 2 years to less than 18 years of age with eGFR 50 mL/min/1.73 m2 or less. There is insufficient information to recommend a dosing regimen for pediatric patients younger than 2 years of age with renal impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
10
OVERDOSAGE
In the event of overdose, discontinue AVYCAZ and institute general supportive treatment.
Ceftazidime and avibactam can be removed by hemodialysis. In subjects with end-stage renal disease (ESRD) administered 1 gram ceftazidime, the mean total recovery in dialysate following a 4-hour hemodialysis session was 55% of the administered dose. In subjects with ESRD administered 100 mg avibactam, the mean total recovery in dialysate following a 4-hour hemodialysis session started 1 hour after dosing was approximately 55% of the dose.
No clinical information is available on the use of hemodialysis to treat AVYCAZ overdosage [see Clinical Pharmacology (12.3)].
-
11
DESCRIPTION
AVYCAZ is an antibacterial combination product consisting of the semisynthetic cephalosporin ceftazidime pentahydrate and the beta-lactamase inhibitor avibactam sodium for intravenous administration.
Ceftazidime
Ceftazidime is a semisynthetic, beta-lactam antibacterial drug. It is the pentahydrate of (6R,7R,Z)-7-(2-(2-aminothiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetamido)-8-oxo-3-(pyridinium-1-ylmethyl)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate. Its molecular weight is 636.6. The empirical formula is C22H32N6O12S2.
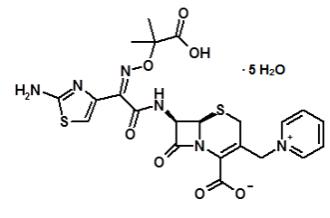
Figure 1. Chemical structure of ceftazidime pentahydrate
Avibactam
Avibactam sodium chemical name is sodium [(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] sulfate. Its molecular weight is 287.23. The empirical formula is C7H10N3O6SNa.
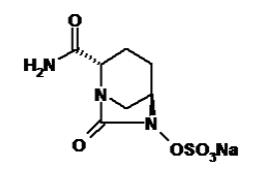
Figure 2. Chemical structure of avibactam sodium
AVYCAZ 2.5 grams (ceftazidime and avibactam) for injection is a white to yellow sterile powder for constitution consisting of ceftazidime pentahydrate and avibactam sodium packaged in glass vials. The formulation also contains sodium carbonate.
Each AVYCAZ 2.5 grams single-dose vial contains ceftazidime 2 grams (equivalent to 2.635 grams sterile ceftazidime pentahydrate/sodium carbonate) and avibactam 0.5 grams (equivalent to 0.551 grams sterile avibactam sodium). The sodium carbonate content of the mixture is 239.6 mg/vial. The total sodium content of the mixture is approximately 146 mg (6.4 mEq)/vial.
-
12
CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
As with other beta-lactam antimicrobial drugs, the time that unbound plasma concentrations of ceftazidime exceed the AVYCAZ minimum inhibitory concentration (MIC) against the infecting organism has been shown to best correlate with efficacy in a neutropenic murine thigh infection model with Enterobacteriaceae and Pseudomonas aeruginosa. The time above a threshold concentration has been determined to be the parameter that best predicts the efficacy of avibactam in in vitro and in vivo nonclinical models.
Cardiac Electrophysiology
In a thorough QT study, a supratherapeutic dose of ceftazidime (3 grams) was investigated for QT effects in combination with a supratherapeutic dose of avibactam (2 grams) given as a 30-minute single infusion. No significant effect on QTcF interval was detected at peak plasma concentration or at any other time. The largest 90% upper bound for the placebo corrected mean change from baseline was 5.9 ms. There were no QTcF intervals greater than 450 ms, nor were there any QTcF interval changes from baseline greater than 30 ms.
12.3 Pharmacokinetics
The mean pharmacokinetic parameters for ceftazidime and avibactam in healthy adult male subjects with normal renal function after single and multiple 2-hour intravenous infusions of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours are summarized in Table 14.
Pharmacokinetic parameters of ceftazidime and avibactam were similar for single and multiple dose administration of AVYCAZ and were similar to those determined when ceftazidime or avibactam were administered alone.
Table 14. Pharmacokinetic Parameters (Geometric Mean [%CV]) of Ceftazidime and Avibactam Following Administration of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) in Healthy Adult Male Subjects Ceftazidime Avibactam Parameter Single AVYCAZ 2.5 gramsa Dose Administered as a 2-hour Infusion (n = 16) Multiple AVYCAZ 2.5 gramsa Doses Administered every 8 hours as 2-hour Infusions for 11 Days (n = 16) Single AVYCAZ 2.5 gramsa Dose
Administered as a 2-hour Infusion
(n = 16)Multiple AVYCAZ 2.5 gramsa Doses
Administered every 8 hours as 2-hour Infusions for 11 Days (n = 16)Cmax (mg/L) 88.1 (14) 90.4 (16) 15.2 (14) 14.6 (17) AUC (mg-h/L)b 289 (15)c 291 (15) 42.1 (16)d 38.2 (19) T1/2 (h) 3.27 (33)c 2.76 (7) 2.22 (31)d 2.71 (25) CL (L/h) 6.93 (15)c 6.86 (15) 11.9 (16)d 13.1 (19) Vss (L) 18.1 (20)c 17 (16) 23.2 (23)d 22.2 (18) CL = plasma clearance; Cmax = maximum observed concentration; T1/2 = terminal elimination half-life; Vss (L) = volume of distribution at steady state
a ceftazidime 2 grams and avibactam 0.5 grams
b AUC0-inf (area under concentration-time curve from time 0 to infinity) reported for single-dose administration; AUC0-tau (area under concentration curve over dosing interval) reported for multiple-dose administration
c n = 15
d n = 13The Cmax and AUC of ceftazidime increase in proportion to dose. Avibactam demonstrated approximately linear pharmacokinetics across the dose range studied (50 mg to 2000 mg) for single intravenous administration. No appreciable accumulation of ceftazidime or avibactam was observed following multiple intravenous infusions of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours for up to 11 days in healthy adults with normal renal function.
Distribution
Less than 10% of ceftazidime was protein bound. The degree of protein binding was independent of concentration. The binding of avibactam to human plasma proteins was low (5.7% to 8.2%) and was similar across the range of concentrations tested in vitro (0.5 to 50 mg/L).
The steady-state volumes of distribution of ceftazidime and avibactam were 17 L and 22.2 L, respectively, in healthy adults following multiple doses of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) infused every 8 hours over 2 hours for 11 days.
Following administration of AVYCAZ 2.5 g (ceftazidime 2 grams and avibactam 0.5 grams) to healthy male subjects every 8 hours as a 2-hour infusion for 3 days, the mean bronchial epithelial lining fluid-to-plasma ratios of avibactam Cmax and AUC0-tau were 35%. The mean bronchial epithelial lining fluid-to-plasma ratios of ceftazidime Cmax and AUC0-tau were 26% and 31%, respectively.
Metabolism
Ceftazidime is mostly (80% to 90% of the dose) eliminated as unchanged drug. No metabolism of avibactam was observed in human liver preparations (microsomes and hepatocytes). Unchanged avibactam was the major drug-related component in human plasma and urine after a single intravenous dose of 0.5 grams 14C-labelled avibactam.
Excretion
Both ceftazidime and avibactam are excreted mainly by the kidneys.
Approximately 80% to 90% of an intravenous dose of ceftazidime is excreted unchanged by the kidneys over a 24-hour period. After the intravenous administration of single 0.5-grams or 1-gram doses, approximately 50% of the dose appeared in the urine in the first 2 hours. An additional 20% was excreted between 2 and 4 hours after dosing, and approximately another 12% of the dose appeared in the urine between 4 and 8 hours later. The elimination of ceftazidime by the kidneys resulted in high therapeutic concentrations in the urine. The mean renal clearance of ceftazidime was approximately 100 mL/min. The calculated plasma clearance of approximately 115 mL/min indicated nearly complete elimination of ceftazidime by the renal route.
Following administration of a single 0.5-grams intravenous dose of radiolabeled avibactam, an average of 97% of administered radioactivity was recovered from the urine, with over 95% recovered within 12 hours of dosing. An average of 0.20% of administered total radioactivity was recovered in feces within 96 hours of dosing. An average of 85% of administered avibactam was recovered from the urine as unchanged drug within 96 hours, with over 50% recovered within 2 hours of the start of the infusion. Renal clearance was 158 mL/min, which is greater than the glomerular filtration, suggesting that active tubular secretion contributes to the excretion of avibactam in addition to glomerular filtration.
Specific Populations
Patients with Renal Impairment
Ceftazidime is eliminated almost solely by the kidneys; its serum half-life is significantly prolonged in patients with impaired renal function.
The clearance of avibactam was significantly decreased in subjects with mild (CrCl greater than 50 to 80 mL/min, n = 6), moderate (CrCl 30 to less than or equal to 50 mL/min, n = 6), and severe (CrCl 30 mL/min or less, not requiring hemodialysis; n = 6) renal impairment compared to healthy subjects with normal renal function (CrCl greater than 80 mL/min, n = 6) following administration of a single 100-mg intravenous dose of avibactam. The slower clearance resulted in increases in systemic exposure (AUC) of avibactam of 2.6-fold, 3.8-fold, and 7-fold in subjects with mild, moderate, and severe renal impairment, respectively, compared to subjects with normal renal function.
A single 100-mg dose of avibactam was administered to subjects with ESRD (n = 6) either 1 hour before or after hemodialysis. The avibactam AUC following the post-hemodialysis infusion was 19.5-fold the AUC of healthy subjects with normal renal function. Avibactam was extensively removed by hemodialysis, with an extraction coefficient of 0.77 and a mean hemodialysis clearance of 9.0 L/h. Approximately 55% of the avibactam dose was removed during a 4-hour hemodialysis session.
Dosage adjustment of AVYCAZ is recommended in adult and pediatric patients 2 years and older with moderate and severe renal impairment and end-stage renal disease. Population PK models for ceftazidime and avibactam were used to conduct simulations for patients with impaired renal function. Simulations demonstrated that the recommended dose adjustments [see Dosage and Administration (2.3)] provide comparable exposures of ceftazidime and avibactam in both adult and pediatric patients with moderate and severe renal impairment and end-stage renal disease to those in patients with normal renal function or mild renal impairment. Because the exposure of both ceftazidime and avibactam is highly dependent on renal function, monitor renal function (i.e., CrCl in adult patients and eGFR in pediatric patients) at least daily and adjust the dosage of AVYCAZ accordingly [see Dosage and Administration (2.3)]. There is insufficient information to recommend a dosage regimen for pediatric patients less than 2 years of age with renal impairment.
Patients with Hepatic Impairment
The presence of hepatic dysfunction had no effect on the pharmacokinetics of ceftazidime in individuals administered 2 grams intravenously every 8 hours for 5 days.
The pharmacokinetics of avibactam in patients with hepatic impairment have not been established. Avibactam does not appear to undergo significant hepatic metabolism; therefore, the systemic clearance of avibactam is not expected to be significantly affected by hepatic impairment.
Dose adjustments are not currently considered necessary for AVYCAZ in patients with impaired hepatic function.
Pediatric Patients
Population pharmacokinetic analyses and target attainment simulations were conducted to recommend AVYCAZ dosing in pediatric patients with cIAI, cUTI and HABP/VABP. The recommended pediatric dosing regimens for patients from 2 to less than 18 years of age with eGFR of 50 mL/min/1.73 m2 or higher and for patients from birth (with gestational age 31 weeks and older) to less than 2 years without renal impairment are predicted to result in systemic exposures similar to that in adult patients given AVYCAZ 2.5 grams. Population PK modeling, including the assumption of proportional effects of renal impairment in adults and pediatric patients, also predicted that the recommended dose adjustments for patients aged 2 years and older with eGFR less than 50 mL/min/1.73 m2 result in systemic exposure similar to that in adult patients. There is insufficient information to recommend a dosage adjustment in pediatric patients less than 2 years of age with renal impairment.
Geriatric Patients
Following single-dose administration of 0.5 grams avibactam as a 30-minute infusion the mean AUC for avibactam was 17% higher in healthy elderly subjects (65 years of age and older, n = 16) than in healthy young adult subjects (18 to 45 years of age, n = 17). There was no statistically significant age effect for avibactam Cmax.
No dose adjustment is recommended based on age. Dosage adjustment for AVYCAZ in elderly patients should be based on renal function [see Dosage and Administration (2.2)].
Gender
Following single-dose administration of 0.5 grams avibactam as a 30-minute infusion, healthy male subjects (n = 17) had 18% lower avibactam Cmax values than healthy female subjects (n = 16). There was no gender effect for avibactam AUC parameters.
No dose adjustment is recommended based on gender.
Drug Interactions
Avibactam at clinically relevant concentrations does not inhibit the cytochrome P450 isoforms CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4/5 in vitro in human liver microsomes. Avibactam showed no potential for in vitro induction of CYP1A2, 2B6, 2C9 and 3A4 isoenzymes in human hepatocytes. Against CYP2E1, avibactam showed a slight induction potential at very high concentrations that exceed any clinically relevant exposure. Ceftazidime was evaluated independently in human hepatocytes and showed no induction potential on the activity or mRNA expression of CYP1A1/2, CYP2B6, and CYP3A4/5.
Neither ceftazidime nor avibactam was found to be an inhibitor of the following hepatic and renal transporters in vitro at clinically relevant concentrations: MDR1, BCRP, OAT1, OAT3, OATP1B1, OATP1B3, BSEP, MRP4, OCT1 and OCT2. Avibactam was not a substrate of MDR1, BCRP, MRP4, or OCT2, but was a substrate of human OAT1 and OAT3 kidney transporters based on results generated in human embryonic kidney cells expressing these transporters. Probenecid inhibits 56% to 70% of the uptake of avibactam by OAT1 and OAT3 in vitro. Ceftazidime does not inhibit avibactam transport mediated by OAT1 and OAT3. The clinical impact of potent OAT inhibitors on the pharmacokinetics of avibactam is not known. Co-administration of AVYCAZ with probenecid is not recommended [see Drug Interactions (7.1)].
Administration of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) to healthy male subjects (n = 28) as a 2-hour infusion following a 1-hour infusion of metronidazole every 8 hours for 3 days did not affect the Cmax and AUC values for avibactam or ceftazidime compared to administration of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) alone. Administration of 0.5 grams metronidazole to healthy male subjects as a 1-hour infusion before a 2-hour infusion of AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) every 8 hours for 3 days did not affect the Cmax and AUC of metronidazole compared to administration of 0.5 grams metronidazole alone.
12.4 Microbiology
Mechanism of Action
The ceftazidime component of AVYCAZ is a cephalosporin antibacterial drug with in vitro activity against certain gram-negative and gram-positive bacteria. The bactericidal action of ceftazidime is mediated through binding to essential penicillin-binding proteins (PBPs). The avibactam component of AVYCAZ is a non-beta-lactam beta-lactamase inhibitor that inactivates certain beta-lactamases that degrade ceftazidime. Avibactam does not decrease the activity of ceftazidime against ceftazidime-susceptible organisms.
AVYCAZ demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and extended-spectrum beta-lactamases (ESBLs) of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase (KPCs), AmpC, and certain oxacillinases (OXA). AVYCAZ also demonstrated in vitro activity against P. aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin (OprD). AVYCAZ is not active against bacteria that produce metallo-beta lactamases and may not have activity against gram-negative bacteria that overexpress efflux pumps or have porin mutations.
Resistance
No cross-resistance with other classes of antimicrobials has been identified. Some isolates resistant to other cephalosporins (including ceftazidime) and to carbapenems may be susceptible to AVYCAZ.
Interaction with Other Antimicrobials
In vitro studies have not demonstrated antagonism between AVYCAZ and colistin, levofloxacin, linezolid, metronidazole, tigecycline, tobramycin, or vancomycin.
Activity against Ceftazidime-Nonsusceptible Bacteria in Animal Infection Models
Avibactam restored activity of ceftazidime in animal models of infection (e.g. thigh infection, pyelonephritis, systemic infection induced by intraperitoneal injection) caused by ceftazidime non-susceptible beta-lactamase- producing (e.g., ESBL, KPC and AmpC) gram-negative bacteria.
Antimicrobial Activity
AVYCAZ has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see Indications and Usage (1.1), (1.2) and (1.3)].
Complicated Intra-abdominal Infections (cIAI)
Aerobic BacteriaGram-negative Bacteria
- Citrobacter freundii complex
- Enterobacter cloacae
- Escherichia coli
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Proteus mirabilis
- Pseudomonas aeruginosa
Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
Aerobic BacteriaGram-negative Bacteria
- Citrobacter freundii complex
-
Enterobacter cloacae
-
Escherichia coli
-
Klebsiella pneumoniae
-
Proteus mirabilis
- Pseudomonas aeruginosa
Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
Aerobic BacteriaGram-negative Bacteria
- Enterobacter cloacae
-
Escherichia coli
-
Haemophilus influenzae
-
Klebsiella pneumoniae
-
Proteus mirabilis
-
Pseudomonas aeruginosa
- Serratia marcescens
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for AVYCAZ against isolates of similar genus or organism group. However, the efficacy of AVYCAZ in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Gram-negative Bacteria
- Citrobacter koseri
-
Enterobacter aerogenes
-
Morganella morganii
-
Providencia rettgeri
- Providencia stuartii
Susceptibility Test Methods
For specific information regarding susceptibility testing methods, interpretive criteria, and associated test methods and quality control standards recognized by FDA for AVYCAZ, please see: https://www.fda.gov/STIC.
- Citrobacter freundii complex
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ceftazidime and avibactam were each evaluated for mutagenic potential in several in vitro and in vivo assays. Ceftazidime was negative for mutagenicity in a mouse micronucleus test and an Ames test. Avibactam was negative for genotoxicity in the Ames assay, unscheduled DNA synthesis, chromosomal aberration assay, and a rat micronucleus study.
Avibactam had no adverse effects on fertility of male and female rats given up to 1 g/kg/day (approximately 20-fold higher than the recommended clinical dose on a body surface area basis). There was a dose-related increase in the percentage of pre- and post-implantation loss relative to controls, resulting in a lower mean litter size at doses 0.5 g/kg and greater with intravenous administration to female rats beginning 2 weeks prior to mating.
-
14
CLINICAL STUDIES
14.1 Complicated Intra-abdominal Infections
Adult Patients
A total of 1058 adults hospitalized with cIAI were randomized and received trial medications in a multinational, multi-center, double-blind trial comparing AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours plus metronidazole (0.5 grams intravenously every 8 hours) to meropenem (1 gram intravenously every 8 hours) for 5 to 14 days of therapy. Complicated intra-abdominal infections included appendicitis, cholecystitis, diverticulitis, gastric/duodenal perforation, perforation of the intestine, and other causes of intra-abdominal abscesses and peritonitis.
The microbiologically modified intent-to treat (mMITT) population, which included all patients who had at least one baseline intra-abdominal pathogen, consisted of 823 patients; the median age was 51 years and 62.8% were male. The majority of patients (64.9%) were from Eastern Europe; 7.5% were from the United States. Less than 1.0% of patients were of Pacific Island or African descent. The most common primary cIAI diagnosis was appendiceal perforation or peri-appendiceal abscess, occurring in 44.7% of patients. Bacteremia at baseline was present in 4.3% of patients.
Clinical cure was defined as complete resolution or significant improvement in signs and symptoms of the index infection at the test-of-cure (TOC) visit which occurred 28 to 35 days after randomization. Table 15 presents the clinical cure in the mMITT population and in the microbiologically evaluable (ME) population, which included all protocol-adherent mMITT patients. AVYCAZ plus metronidazole was non-inferior to meropenem with regard to the primary endpoint (clinical cure rate at the TOC visit in the mMITT population). Clinical cure rates at the TOC visit by pathogen in the mMITT population are presented in Table 16.
Table 15. Clinical Cure Rates at TOC from the Phase 3 cIAI Trial Analysis population AVYCAZ plus metronidazolea
n/N (%)Meropenemb
n/N (%)Treatment Difference
(95% CI)cmMITT 337/413 (81.6) 349/410 (85.1) -3.5 (-8.6, 1.6) ME 244/265 (92.1) 272/287 (94.8) -2.7 (-7.1, 1.5) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours + metronidazole 0.5 grams IV every 8 hours
b 1 gram IV every 8 hours
c The 95% confidence interval (CI) was calculated as an unstratified Miettinen and Nurminen methodOf the 823 patients in the mMITT population, 14 (1.7%) had baseline E. coli bacteremia; 7/10 (70.0%) of patients in the AVYCAZ arm and 3/4 (75.0%) of patients in the meropenem arm had a clinical cure.
Table 16. Clinical Cure Rates at TOC by Baseline Pathogen from the Phase 3 cIAI Trial, mMITT Population Aerobic Gram-negative group or pathogen AVYCAZ plus metronidazolea
n/N (%)Meropenemb
n/N (%)Enterobacteriaceae 272/334 (81.4) 305/353 (86.4) Escherichia coli 218/271 (80.4) 248/285 (87.0) Klebsiella pneumoniae 40/51 (78.4) 37/49 (75.5) Klebsiella oxytoca 14/18 (77.8) 12/15 (80.0) Enterobacter cloacae 11/13 (84.6) 16/19 (84.2) Citrobacter freundii complex 14/18 (77.8) 9/12 (75.0) Proteus mirabilis 5/8 (62.5) 7/9 (77.8) Pseudomonas aeruginosa 30/35 (85.7) 34/36 (94.4) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours + metronidazole 0.5 grams IV every 8 hours
b 1 gram IV every 8 hoursAt baseline, 111 patients in the mMITT population had Gram-negative isolates that were not susceptible to ceftazidime, including 61 patients with E. coli and 26 patients with K. pneumoniae isolates. Cure rates were 39/47 (83.0%) in patients who received AVYCAZ and 55/64 (85.9%) of patients who received meropenem.
In a subset of Gram-negative pathogens from both arms of the Phase 3 cIAI trial that met phenotypic screening criteria for the presence of a beta-lactamase, genotypic testing identified certain ESBL groups (e.g., TEM-1, SHV-12, CTX-M-15, OXA-48) and AmpC that were expected to be inhibited by avibactam in isolates from 105 (12.8%) of the 823 patients in the mMITT population. Clinical cure rates in this subset were similar to the overall results.
Pediatric Patients
The pediatric cIAI trial was a randomized, single-blind, multi-center, active controlled trial conducted in hospitalized patients aged 3 months to less than 18 years. Patients were randomized in a 3:1 ratio to receive either AVYCAZ [see Dosage and Administration (2.2)] plus metronidazole (10 mg/kg IV over 20 to 30 minutes every 8 hours), or meropenem (20 mg/kg IV every 8 hours). Patients received IV treatment for a minimum of 72 hours before an optional switch to oral therapy at the discretion of the investigator to complete a total of 7 to 15 days of antibacterial therapy.
The intent-to treat (ITT) population consisted of 83 patients (AVYCAZ plus metronidazole, n=61, meropenem n=22) who were randomized to receive treatment; 64% were male, and the median age was 11.0 years in the AVYCAZ plus metronidazole group (range 3 to 17 years). The pediatric age groups who received AVYCAZ plus metronidazole were as follows: 12 to <18 years, (n=22), 6 to < 12 years, (n=33), 3 to < 6 years (n=6). No patients less than 2 years of age received AVYCAZ plus metronidazole. Most patients (87%) had a diagnosis of appendiceal perforation or peri-appendiceal abscess. The microbiological intent-to treat (micro-ITT) population, which included all patients who had at least one baseline intra-abdominal pathogen, consisted of 69 patients (AVYCAZ plus metronidazole, n=50; meropenem, n=19). The predominant baseline pathogens were E. coli (79.7%) and P. aeruginosa (33.3%).
The primary objective of the study was to evaluate the safety and tolerability of AVYCAZ and it was not powered for a statistical analysis of efficacy. At the TOC visit, which occurred 8 to 15 days after the last dose of study drug, a favorable clinical response was defined as the resolution of all acute signs and symptoms of cIAI or improvement to such an extent that no further antimicrobial therapy was required. The clinical cure rates for the trial at TOC are described in Table 17.
Table 17. Clinical Cure Rates at TOC from the Pediatric cIAI Trial Analysis population AVYCAZ plus metronidazolea
n/N (%)Meropenemb
n/N (%)ITT 56/61 (91.8) 21/22 (95.5) Micro-ITT 45/50 (90.0) 18/19 (94.7) a AVYCAZ doses as per Table 2, Dosage and Administration + metronidazole 10 mg/kg IV every 8 hours
b 20 mg/kg IV every 8 hoursClinical cure rates for the predominant pathogens, E.coli and P.aeruginosa, were 90.5% and 85.7%, respectively for patients treated with AVYCAZ plus metronidazole, and 92.3% and 88.9%, respectively, for patients treated with meropenem.
14.2 Complicated Urinary Tract Infections, Including Pyelonephritis
Adult Patients
The efficacy of AVYCAZ in patients with cUTI was evaluated in two randomized, actively controlled clinical trials (Trial 1 and Trial 2) as described below.
cUTI Trial 1
A total of 1020 adults hospitalized with cUTI were randomized and received trial medications in a multinational, multi-center, double-blind trial comparing AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours to doripenem 0.5 grams intravenously every 8 hours for 10 to 14 days of total therapy. A switch to an oral antimicrobial agent was allowed after 5 days of intravenous dosing. Complicated urinary tract infections included acute pyelonephritis and complicated lower urinary tract infections.
The mMITT population, which included all patients who had at least one uropathogen isolated at baseline (greater or equal to 105 CFU/mL), consisted of 810 patients; the median age was 55 years and 69.8% were female. The majority of patients (75.4%) were from Eastern Europe; less than 1% of patients were from the United States. The majority of patients were White (83%) or Asian (7.8%); other racial subgroups were each represented at less than 1%. The most common diagnosis was acute pyelonephritis, occurring in 72% of patients. Bacteremia was present at baseline in 8.8% of patients.
Clinical efficacy was determined by comparing the response rate of AVYCAZ to doripenem at both primary endpoints; symptom response rates at Day 5 and combined microbiological cure and symptom response rates at the TOC visit (21 to 25 days after randomization). A symptom response was based on the resolution of patient-reported cUTI symptoms, defined as frequency/urgency/dysuria/suprapubic pain, as well as an improvement in flank pain for individuals with acute pyelonephritis. Microbiological cure was defined as a reduction of all baseline uropathogens to less than 104 CFU/mL in the urine.
AVYCAZ was non-inferior to doripenem with regard to both primary endpoints as presented in Table 18.
Table 18. Clinical and Microbiological Cure Rates from cUTI Trial 1, mMITT Population Study endpoint AVYCAZa
n/N (%)Doripenemb
n/N (%)Treatment Difference
(95% CI)cSymptomatic response at Day 5 276/393 (70.2) 276/417 (66.2) 4.0 (-2.4, 10.4) Combined symptomatic and microbiological response at TOC 280/393 (71.2) 269/417 (64.5) 6.7 (0.3, 13.1) Microbiological cure at TOC 304/393 (77.4) 296/417 (71.0) 6.4 (0.3, 12.4) Symptomatic response at TOC 332/393 (84.5) 360/417 (86.3) -1.9 (-6.8, 3.0) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b 0.5 grams IV every 8 hours
c The 95% confidence interval (CI) was calculated using the unstratified Miettinen and Nurminen methodMicrobiological cure rates by pathogen are presented in Table 19. Microbiological cure in individuals with bacteremia at baseline was achieved in 31/38 (81.6%) patients in the AVYCAZ arm and 24/33 (72.7%) patients in the doripenem arm at the TOC visit in the mMITT population. The most common pathogen isolated from blood was Escherichia coli, for which 31/32 (96.9%) patients in the AVYCAZ arm were microbiological cures, compared with 28/28 (100%) patients in the doripenem arm.
Table 19. Microbiological Cure Rate at TOC by Baseline Pathogen from cUTI Trial 1, mMITT Population Aerobic Gram-negative group or pathogen AVYCAZa
n/N (%)Doripenemb
n/N (%)Enterobacteriaceae 299/382 (78.3) 281/398 (70.6) Escherichia coli 229/292 (78.4) 220/306 (71.9) Klebsiella pneumoniae 33/44 (75.0) 35/56 (62.5) Proteus mirabilis 16/17 (94.1) 9/13 (69.2) Enterobacter cloacae 6/11 (54.5) 9/13 (69.2) Pseudomonas aeruginosa 12/18 (66.7) 15/20 (75.0) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b 0.5 grams IV every 8 hoursAt baseline, 159 patients in the mMITT population had Gram-negative isolates that were not susceptible to ceftazidime, including 75 patients in the AVYCAZ arm and 84 in the doripenem arm. Microbiological and clinical cure rates at TOC were 47/75 (62.7%) and 67/75 (89.3%), respectively, in patients who received AVYCAZ, compared to 51/84 (60.7%) and 75/84 (89.3%) in patients who received doripenem.
In a subset of Gram-negative pathogens from both arms of the Phase 3 cUTI trial that met phenotypic screening criteria for the presence of a beta-lactamase, genotypic testing identified certain ESBL groups (e.g., TEM-1, SHV-12, CTX-M-15, CTX-M-27, OXA-48) and AmpC that were expected to be inhibited by avibactam in isolates from 176 (21.7%) of the 810 patients in the mMITT population. Microbiological and clinical cure rates in this subset were similar to the overall trial results.
cUTI Trial 2
In a multinational, multi-center, open-label study of adults hospitalized with ceftazidime non-susceptible (CAZ-NS) Gram-negative infections, 305 patients with cUTI were randomized and received AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours or the best available intravenous therapy (BAT) for 5 to 21 days of treatment. There was no optional switch to oral therapy. The majority (96.1%) of patients in the BAT arm received monotherapy with a carbapenem antibacterial drug. Complicated urinary tract infections included acute pyelonephritis and complicated lower urinary tract infections.
The mMITT population consisted of 281 cUTI patients with at least one baseline CAZ-NS uropathogen (defined as MIC greater or equal to 8 mg/L for Enterobacteriaceae and greater or equal to 16 mg/L for P. aeruginosa). The median age was 65 years and 54.8% were male. The majority of cUTI patients (82.2%) were from Eastern Europe; 2.8% were from the United States. The majority of patients (95%) were White. The most common diagnosis was cUTI without pyelonephritis, occurring in 54.8% of patients. Bacteremia at baseline was present in 3.6% of patients.
Clinical efficacy was based on evaluation of both the clinical cure (defined as resolution or significant improvement of baseline cUTI signs and symptoms) and microbiological cure (all baseline uropathogens were reduced to less than 104 CFU/mL) rates at the follow-up visit (21 to 25 calendar days from randomization) in the mMITT population.
The clinical and microbiological response rates at the follow-up visit in the mMITT population are presented in Table 20. The microbiological response rates at the follow-up visit by baseline CAZ-NS uropathogen in the mMITT population are presented in Table 21.
Table 20. Clinical and Microbiological Response Rates at Day 21 to 25 visit from Trial 2 (cUTI Patients), mMITT Population Study Endpoint AVYCAZa
n/N (%)BATb
n/N (%)
Treatment Difference
(95% CI)cCombined clinical and microbiological cure 101/144 (70.1) 74/137 (54.0) 16.1 (4.8, 27.1) Clinical cure 127/144 (88.2) 121/137 (88.3) -0.1 (-7.9, 7.7) Microbiological cure 103/144 (71.5) 78/137 (56.9) 14.6 (3.4, 25.5) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b Best available therapy (BAT) options were meropenem, imipenem, doripenem, and colistin; the majority of patients received carbapenem monotherapy
c The 95% confidence interval (CI) was calculated using the unstratified Miettinen and Nurminen methodTable 21. Microbiological Response Rates by Baseline CAZ-NS Pathogen at the Day 21 to 25 visit from Trial 2 (cUTI Patients), mMITT Population Aerobic Gram-negative pathogen AVYCAZa
n/N (%)BATb
n/N (%)Enterobacteriaceae Escherichia coli 45/59 (76.3) 33/57 (57.9) Klebsiella pneumoniae 42/55 (76.4) 39/65 (60.0) Pseudomonas aeruginosa 8/14 (57.1) 3/5 (60.0) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b Best available therapy (BAT) options were meropenem, imipenem, doripenem, and colistin; the majority of patients received carbapenem monotherapyAmong Gram-negative uropathogens from both arms of Trial 2, genotypic testing identified certain ESBL groups (e.g., TEM-1, SHV-12, CTX-M-15, CTX-M-27, KPC-2, KPC-3, OXA-48) and AmpC beta-lactamases expected to be inhibited by avibactam in isolates from 273/281 (97.2%) patients in the mMITT population. Clinical and microbiological cure rates in this subset were similar to the overall results.
Pediatric Patients
The cUTI pediatric trial was a randomized, single-blind, multi-center, active-controlled study conducted in hospitalized patients aged 3 months to less than 18 years. Patients were randomized in a 3:1 ratio to receive either AVYCAZ [see Dosage and Administration (2.2)] or cefepime (dosed per local standard of care, and not to exceed 2000 mg per infusion). Patients received IV treatment for a minimum of 72 hours before an optional switch to oral therapy at the discretion of the investigator to complete a total of 7 to 14 days of antibacterial therapy.
A study population of 95 patients with cUTI received study medication (AVYCAZ, n=67, cefepime n=28); 81% were female, and the median age was 4.2 years in the AVYCAZ group (range 3.5 months to 18 years). The pediatric age groups who received AVYCAZ were as follows: 12 to <18 years, (n=13), 6 to < 12 years, (n=17), 2 to < 6 years (n=11), 1 to < 2 years (n=12), and 3 months to < 1 year of age (n=14). Most patients had a diagnosis of acute pyelonephritis (83%). The micro-ITT population consisted of 77 patients with at least one Gram-negative uropathogen at baseline (greater or equal to 105 CFU/mL). The predominant baseline pathogen was E. coli (92.2%).
The primary objective of the study was to evaluate the safety and tolerability of AVYCAZ and it was not powered for a statistical analysis of efficacy. At the TOC visit, which occurred 8 to 15 days after the last dose of study drug, a favorable clinical response was defined as a resolution of all acute signs and symptoms of cUTI or improvement to such an extent that no further antimicrobial therapy was required. A favorable microbiological response at the TOC was defined as eradication of baseline uropathogen(s) from the urine culture.
A summary of clinical, microbiological and combined response at TOC by treatment group for the micro-ITT population is provided in Table 22.
Table 22. Clinical and Microbiological Response Rates from the Pediatric cUTI Trial, micro-ITT Population Study Endpoint AVYCAZa
n/N (%)Cefepimeb
n/N (%)
Combined clinical and microbiological cure 39/54 (72.2) 14/23 (60.9) Clinical cure 48/54 (88.9) 19/23 (82.6) Microbiological cure 43/54 (79.6) 14/23 (60.9) a AVYCAZ doses as per Table 2, [see Dosage and Administration (2.2)]
b Dosed per local standard of care, and did not exceed 2000 mgThe microbiologic response rate for E.coli, the most common uropathogen identified in the study, was 79.6% for patients treated with AVYCAZ and 59.1% for patients treated with cefepime.
14.3 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia
A total of 870 hospitalized adults with HABP/VABP were randomized and received trial medications in a multinational, multi-center, double-blind trial comparing AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours to meropenem 1 gram intravenously every 8 hours for 7 to 14 days of therapy. Study medication dosages were adjusted per renal function. The protocol allowed for administration of prior and concomitant systemic antibacterial therapy.
Clinical efficacy was evaluated in the intent-to treat (ITT) population, which included all randomized patients who received study drug. The median age was 66 years and 74.1% were male. The median APACHE II score was 14. The majority of patients were from China (33.1%) and Eastern Europe (25.5%). There were no patients enrolled within the United States. Less than 1.0% of patients were of Pacific Island or African descent. Overall, 379 (43.6%) patients were ventilated at enrollment, including 290 (33.3%) patients with VABP and 89 (10.2%) with ventilated HABP. Bacteremia at baseline was present in 4.8% of patients.
In the AVYCAZ and meropenem treatment groups up to 26% of patients received more than 24 hours of potentially effective systemic Gram-negative antibacterial therapy in the 3 days prior to randomization. Patients with infections only due to Gram-positive organisms were excluded from the trial, when this could be determined before enrollment. Following randomization, patients in both treatment groups could receive empiric open-label linezolid or vancomycin to cover for Gram-positive pathogens while awaiting culture results. Treatment with Gram-positive coverage continued in patients with Gram-positive pathogens.
Adjunctive Gram-negative antibacterial therapy with amikacin or another aminoglycoside was permitted if resistance to meropenem was suspected. Systemic Gram-negative antibacterial therapy was administered to 87% and 86% of patients in the AVYCAZ and meropenem treatment groups, respectively, at any point up to the end of therapy. In either treatment group, up to 36% of patients received more than 72 hours of potentially effective concomitant therapy.
Table 23 presents the 28-day all-cause mortality rates (28 to 32 days after randomization). Results are presented for the ITT population and for the microbiological intent-to-treat (micro-ITT) population, which included all patients with positive culture results indicating the presence of at least one Gram-negative pathogen. Clinical cure at the TOC visit (21-25 days from randomization) is also presented. Clinical cure was defined as resolution or significant improvement in signs and symptoms associated with pneumonia and cessation of antibacterial treatment for HABP/VABP. AVYCAZ was non-inferior to meropenem with regard to the primary endpoint (28-day all-cause mortality in the ITT population). The control group mortality rates were lower than that observed in other HABP/VABP trials which may impact generalizability of results. However, review of patient characteristics reflecting disease severity indicates the study enrolled a representative HABP/VABP population.
Table 23. 28-Day All-cause Mortality and Clinical Cure Rates from the Phase 3 HABP/VABP Trial, ITT and micro-ITT Populations Study Endpoint (Population) AVYCAZa
n/N (%)Meropenemb
n/N (%)Treatment Difference
(95% CI)c28-Day all-cause mortality (ITT) 42/436 (9.6) 36/434 (8.3) 1.5 (- 2.4, 5.3) c micro-ITT 22/187 (11.8) 19/195 (9.7) 2.1 (- 4.1, 8.4) c Clinical cure (ITT) 293/436 (67.2) 300/434 (69.1) - 1.9 (- 8.1, 4.3) d, e a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b 1 gram IV every 8 hours
c The 95% confidence interval (CI) was calculated based on Greenwood’s variance estimates.
d A quantitative estimate of treatment effect has not been established for the clinical cure endpoint.
e The 95% confidence interval (CI) was calculated using an unstratified Miettinen and Nurminen method.The administration of prior or concomitant Gram-negative antibacterial therapy can confound the assessment of trial results. However, a subgroup analysis of 28-day all-cause mortality in subjects who received 24 hours or less of potentially effective antibacterial therapy prior to randomization and 72 hours or less of concomitant potentially effective antibacterial therapy following randomization produced results similar to the overall ITT population, AVYCAZ mortality 10.0% (20/200), meropenem 6.2% (12/195) [difference 3.8%; 95% CI: -1.6 % to 9.5%]). In the subset of patients who received more than 24 hours of potentially effective antibacterial therapy prior to randomization or more than 72 hours of concomitant potentially effective antibacterial therapy following randomization, results were similar to the overall ITT population (AVYCAZ 9.7% (25/258), meropenem 10.5% (28/266) [difference -0.08%; 95% CI: (-6.1% to 4.4%)]).
All-cause mortality rates by pathogen are presented in Table 24. Of the 382 patients in the micro-ITT population, 36 patients were bacteremic at baseline; 20/21 (95.2%) in the AVYCAZ arm and 13/15 (86.7%) in the meropenem arm survived through the day-28 follow-up visit; 13/21 (61.9%) patients in the AVYCAZ arm and 9/15 (60%) of patients in the meropenem arm had a clinical cure at the TOC visit.
The 28-day all-cause mortality rates by pathogen in the micro-ITT population are presented in Table 24. The clinical cure rates at TOC by pathogen in the micro-ITT population are presented in Table 25.
Table 24. 28-Day All-cause Mortality by Baseline Pathogen from the Phase 3 HABP/VABP Trial, micro-ITT Population Aerobic Gram-negative group or pathogen AVYCAZa
n/N (%)Meropenemb
n/N (%)Enterobacteriaceae Klebsiella pneumoniae 11/65 (16.9) 9/75 (12.0) Enterobacter cloacae 0/29 (0) 4/23 (17.4) Escherichia coli 4/22 (18.2) 3/23 (13.0) Serratia marcescens 0/15 (0) 0/13 (0) Proteus mirabilis 1/14 (7.1) 1/12 (8.3) Haemophilus influenzae 1/16 (6.3) 2/25 (8.0) Pseudomonas aeruginosa 9/64 (14.1) 4/51 (7.8) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b 1 gram IV every 8 hoursTable 25. Clinical Cure Rates at TOC by Baseline Pathogen from the Phase 3 HABP/VABP Trial, micro-ITT Population Aerobic Gram-negative group or pathogen AVYCAZa
n/N (%)Meropenemb
n/N (%)Enterobacteriaceae 92/133 (69.2) 108/147 (73.5) Klebsiella pneumoniae 44/65 (67.7) 56/75 (74.7) Enterobacter cloacae 25/29 (86.2) 13/23 (56.5) Escherichia coli 12/22 (54.5) 17/23 (73.9) Serratia marcescens 11/15 (73.3) 12/13 (92.3) Proteus mirabilis 12/14 (85.7) 9/12 (75.0) Haemophilus influenzae 13/16 (81.3) 20/25 (80.0) Pseudomonas aeruginosa 38/64 (59.4) 37/51 (72.5) a AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) IV every 8 hours
b 1 gram IV every 8 hoursAt baseline, 108/382 (28.3%) of patients in the micro-ITT population had Gram-negative isolates that were not susceptible to ceftazidime, including 53 patients with K. pneumoniae and 28 patients with P. aeruginosa isolates. The 28-day all-cause mortality in patients with ceftazidime non-susceptible Gram-negative isolates was 8.2% (4/49) in the AVYCAZ arm and 8.5% (5/59) in the meropenem arm. Clinical cure rates at TOC were 37/49 (75.5%) in patients who received AVYCAZ and 42/59 (71.2%) in patients who received meropenem.
-
16
HOW SUPPLIED/STORAGE AND HANDLING
AVYCAZ 2.5 grams (ceftazidime and avibactam) for injection is supplied in single-dose, clear glass vial containing: ceftazidime 2 grams (equivalent to 2.635 grams of ceftazidime pentahydrate/sodium carbonate) and avibactam 0.5 grams (equivalent to 0.551 grams of avibactam sodium). Vials are supplied as individual vial (NDC# 0456-2700-01) and in cartons containing 10 vials (NDC# 0456-2700-10).
AVYCAZ vials should be stored at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature]. Protect from light. Store in carton until time of use.
Storage conditions for constituted and diluted solutions of AVYCAZ for injection are described in another section of labeling [see Dosage and Administration (2.4, 2.6)].
-
17
PATIENT COUNSELING INFORMATION
Serious Allergic Reactions
Advise patients, their families, or caregivers that allergic reactions, including serious allergic reactions, could occur that require immediate treatment. Ask them about any previous hypersensitivity reactions to AVYCAZ, other beta-lactams (including cephalosporins), or other allergens [see Warnings and Precautions (5.2)].
Potentially Serious Diarrhea
Advise patients, their families, or caregivers that diarrhea is a common problem caused by antibacterial drugs. Sometimes, frequent watery or bloody diarrhea may occur and may be a sign of a more serious intestinal infection. If severe watery or bloody diarrhea develops, tell them to contact his or her healthcare provider [see Warnings and Precautions (5.3)].
Nervous System Reactions
Advise patients, their families, or caregivers that neurological adverse reactions can occur with AVYCAZ use. Instruct patients their families, or caregivers to inform a healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, and seizures, for immediate treatment, dosage adjustment, or discontinuation of AVYCAZ [see Warnings and Precautions (5.4)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including AVYCAZ should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When AVYCAZ is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by AVYCAZ or other antibacterial drugs in the future [see Warnings and Precautions (5.5)].
Distributed by:
AbbVie, Inc.
North Chicago, IL 60064, USAManufactured by:
ACS Dobfar SpA
Via Alessandro Fleming, 2
Verona 37135, ItalyAVYCAZ® and its designs are trademarks of Allergan Sales, LLC, an AbbVie company.
© 2024 AbbVie. All rights reserved.F50000402009
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel -Carton
NDC 0456-2700-10 Rx only 10 Single-dose vials
Avycaz® 2.5 g per vial*
(ceftazidime and avibactam) for injection
*Ceftazidime 2 gram (equivalent to 2.635 g ceftazidime pentahydrate/sodium carbonate)
and avibactam 0.5 g (equivalent to 0.551 g avibactam sodium).
MUST BE CONSTITUTED THEN DILUTED. FOR INTRAVENOUS INFUSION.
-
INGREDIENTS AND APPEARANCE
AVYCAZ
ceftazidime, avibactam powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0456-2700 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFTAZIDIME (UNII: 9M416Z9QNR) (CEFTAZIDIME ANHYDROUS - UNII:DZR1ENT301) CEFTAZIDIME ANHYDROUS 2 g AVIBACTAM SODIUM (UNII: 9V824P8TAI) (AVIBACTAM - UNII:7352665165) AVIBACTAM 0.5 g Inactive Ingredients Ingredient Name Strength SODIUM CARBONATE (UNII: 45P3261C7T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0456-2700-10 10 in 1 CARTON 12/26/2014 1 NDC:0456-2700-01 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206494 12/26/2014 Labeler - Allergan, Inc. (144796497)

