UP AND UP TEMPORARY MINOR ARTHRITIS PAIN RELIEF- acetaminophen tablet, film coated, extended release
Target Corporation
----------
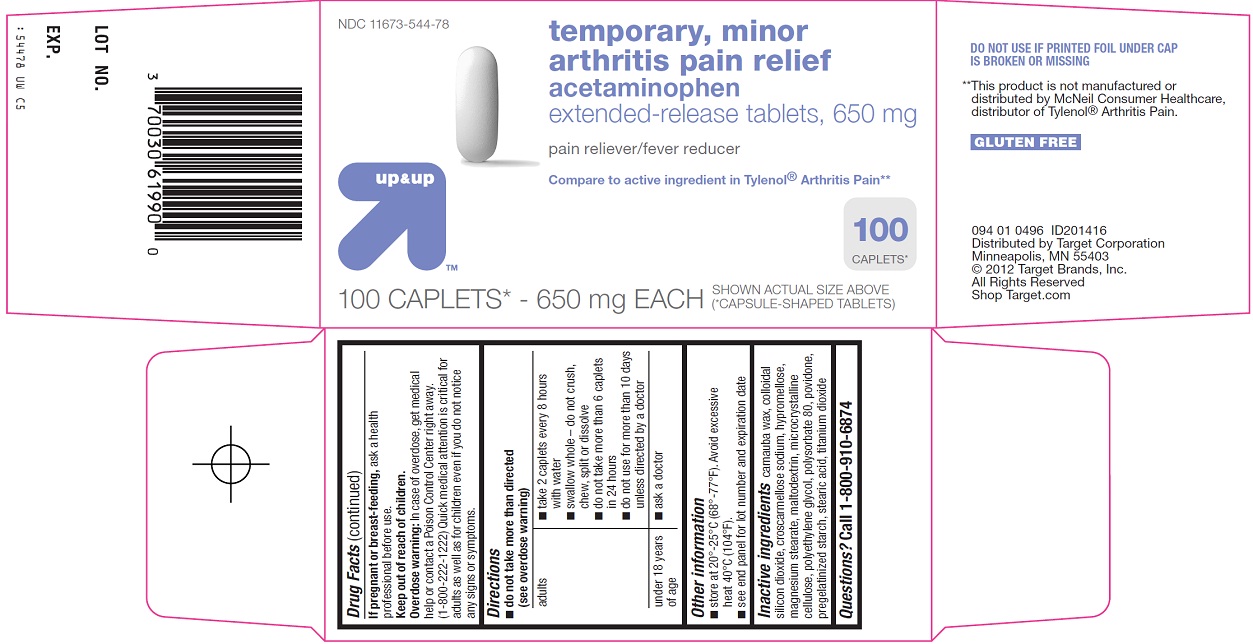
Target Corporation Temporary, Minor Arthritis Pain Relief Drug Facts
Uses
- •
- temporarily relieves minor aches and pains due to:
- •
- minor pain of arthritis
- •
- muscular aches
- •
- backache
- •
- premenstrual and menstrual cramps
- •
- the common cold
- •
- headache
- •
- toothache
- •
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 6 caplets in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- •
- do not take more than directed (see overdose warning)
|
adults |
|
|
under 18 years of age |
|
Other information
- •
- store at 20°-25°C (68°-77°F). Avoid excessive heat 40°C (104°F).
- •
- see end panel for lot number and expiration date
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, stearic acid, titanium dioxide
Principal Display Panel
temporary, minor arthritis pain relief
acetaminophen
extended-release tablets, 650 mg
pain reliever/fever reducer
Compare to active ingredient in Tylenol® Arthritis Pain
# CAPLETS* (replace "#" with actual number of tablets in package)
650 mg EACH
SHOWN ACTUAL SIZE ABOVE
(*CAPSULE-SHAPED TABLETS)
| UP AND UP TEMPORARY MINOR ARTHRITIS PAIN RELIEF
acetaminophen tablet, film coated, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Target Corporation (006961700) |