TRIAMINIC INFANTS FEVER REDUCER PAIN RELIEVER GRAPE- acetaminophen syrup
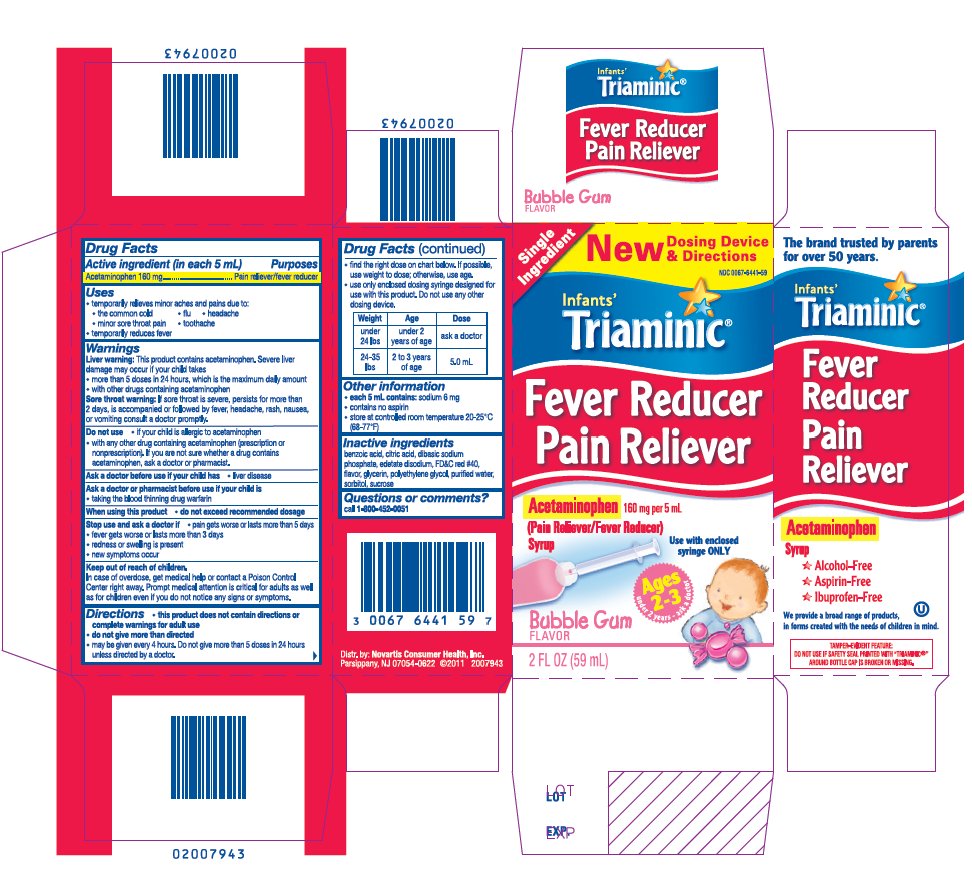
TRIAMINIC INFANTS FEVER REDUCER PAIN RELIEVER BUBBLEGUM- acetaminophen syrup
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- temporarily relieves minor aches and pains due to:
- •
- the common cold
- •
- flu
- •
- headache
- •
- minor sore throat pain
- •
- toothache
- •
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- •
- more than 5 doses in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly.
Do not use
- •
- if your child is allergic to acetaminophen
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
- •
- this product does not contain directions or complete warnings for adult use
- •
- do not give more than directed
- •
- may be given every 4 hours. Do not give more than 5 doses in 24 hours unless directed by a doctor.
- •
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- •
- use only enclosed dosing syringe designed for use with this product. Do not use any other dosing device.
|
Weight |
Age |
Dose |
|
Under 24 lbs |
under 2 years of age |
ask a doctor |
|
24-35 lbs |
2 to 3 years of age |
5.0 mL |
Other information
- •
- each teaspoonful contains: sodium 5 mg <for grape flavor>, sodium 6 mg <for bubblegum flavor>
- •
- contains no aspirin
- •
- store at controlled room temperature 20-25°C (68-77°F)
Inactive ingredients
<for grape flavor>
citric acid, edetate disodium, FD&C blue #1, FD&C red #40, flavor, glycerin, polyethylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol, sucrose
<for bubblegum flavor>
benzoic acid, citric acid, dibasic sodium phosphate, edetate disodium, FD&C red #40, flavor, glycerin, polyethylene glycol, purified water, sorbitol, sucrose
Additional Information Listed on Other Panels
Infants’
Triaminic
Syrup
Fever Reducer
Pain Reliever
Acetaminophen 160 mg per 5 mL
(Pain Reliever/Fever Reducer)
Ages 2-3
Under 2 years-ask a doctor
Use with enclosed syringe ONLY
TAMPER-EVIDENT FEATURE: DO NOT USE IF SAFETY SEAL PRINTED WITH “TRIAMINIC”
AROUND BOTTLE CAP IS BROKEN OR MISSING.
- •
- Alcohol-Free
- •
- Aspirin-Free
- •
- Ibuprofen-Free
- 1.
- We provide a broad range of products, in forms created with the needs of children in mind.
Distr. by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622 ©20XX
| TRIAMINIC
INFANTS FEVER REDUCER PAIN RELIEVER GRAPE
acetaminophen syrup |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| TRIAMINIC
INFANTS FEVER REDUCER PAIN RELIEVER BUBBLEGUM
acetaminophen syrup |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |