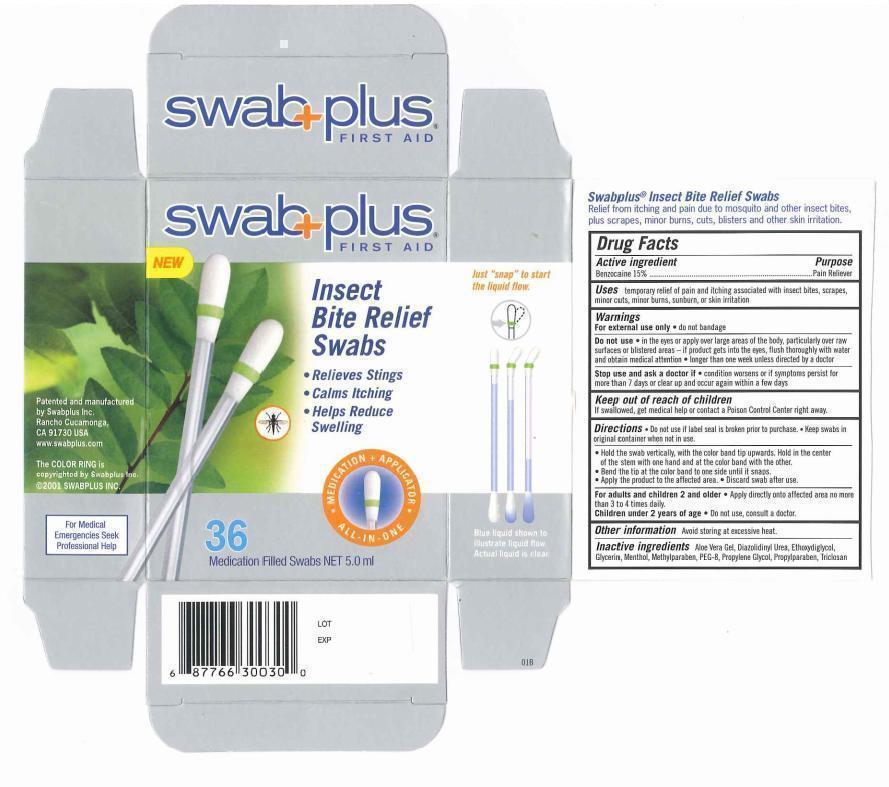
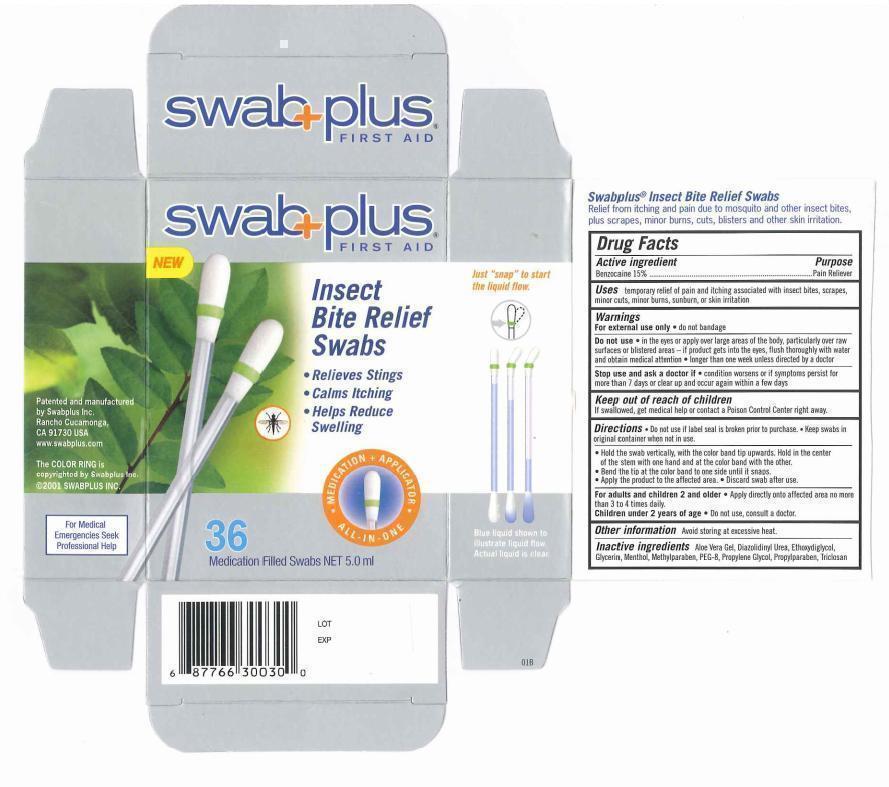
Label: INSECT BITE RELIEF- benzocaine solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 65734-300-00, 65734-300-36 - Packager: Swabplus Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 12, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Fact
- Purpose
- Uses
-
Warnings
For external use only. do not bandage.
Do not use. in the eyes or apply over large areas of the body, particularly over raw surfaces or blistered area - if product gets into the eyes. flush throughly with water and obtain medical attention. longer than one week unless directed by doctor.
Stop use and ask a doctor if. condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days.
- Keep out of reach of children
-
Directions
♦ Do not use if label seal is broken prior to purchase. Keep swabs in original container when not in use.
♦ Hold the swab vertically, with the color band tip upwards. hold in the center of the stem with one hand and at the color band with the other.
♦ Bend the tip at the color band to one side until it snaps.
♦ Apply the product to the affected area.
♦ Discard swab after use.
- Administration
- Other information
- Inactive ingredients
- Package Display Panel
-
INGREDIENTS AND APPEARANCE
INSECT BITE RELIEF
benzocaine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65734-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength Polyethylene Glycol 400 (UNII: B697894SGQ) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) Glycerin (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) Triclosan (UNII: 4NM5039Y5X) Menthol (UNII: L7T10EIP3A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65734-300-36 36 in 1 PACKAGE 1 NDC:65734-300-00 0.15 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 03/01/2003 Labeler - Swabplus Inc. (876441549) Registrant - Swabplus Inc. (876441549) Establishment Name Address ID/FEI Business Operations Swabplus Inc. 876441549 manufacture(65734-300) , relabel(65734-300) , repack(65734-300)