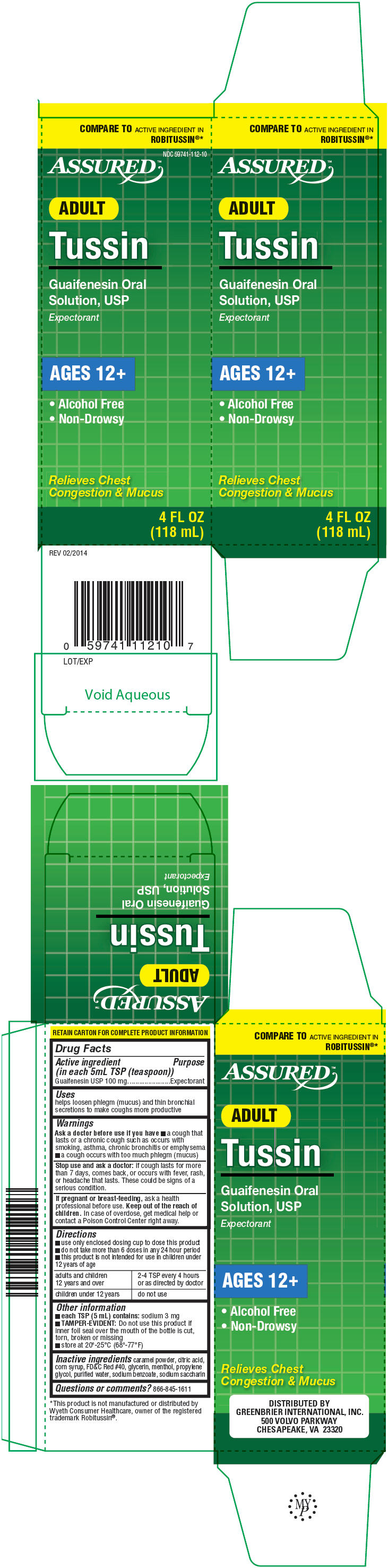
TUSSIN- guaifenesin solution
Bio-Pharm, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Tussin
Warnings
Ask a doctor before use if you have
- a cough that lasts or a chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- a cough occurs with too much phlegm (mucus)
Directions
- use only enclosed dosing cup to dose this product
- do not take more than 6 doses in any 24 hour period
- this product is not intended for use in children under 12 years of age
| adults and children 12 years and over | 2-4 TSP every 4 hours or as directed by doctor |
| children under 12 years | do not use |
Other information
- each TSP (5 mL) contains: sodium 3 mg
- TAMPER-EVIDENT: Do not use this product if inner foil seal over the mouth of the bottle is cut, torn, broken or missing
- store at 20°-25°C (68°-77°F)
| TUSSIN
guaifenesin solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Bio-Pharm, Inc. (801652546) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bio-Pharm, Inc. | 801652546 | MANUFACTURE(59741-112) , ANALYSIS(59741-112) , PACK(59741-112) , LABEL(59741-112) | |
Revised: 8/2018
Document Id: 985ceddb-677c-431a-a14f-8f621a1ca95a
Set id: d629b885-abfd-44ca-980e-c867d4fdbb8e
Version: 2
Effective Time: 20180824
Bio-Pharm, Inc.