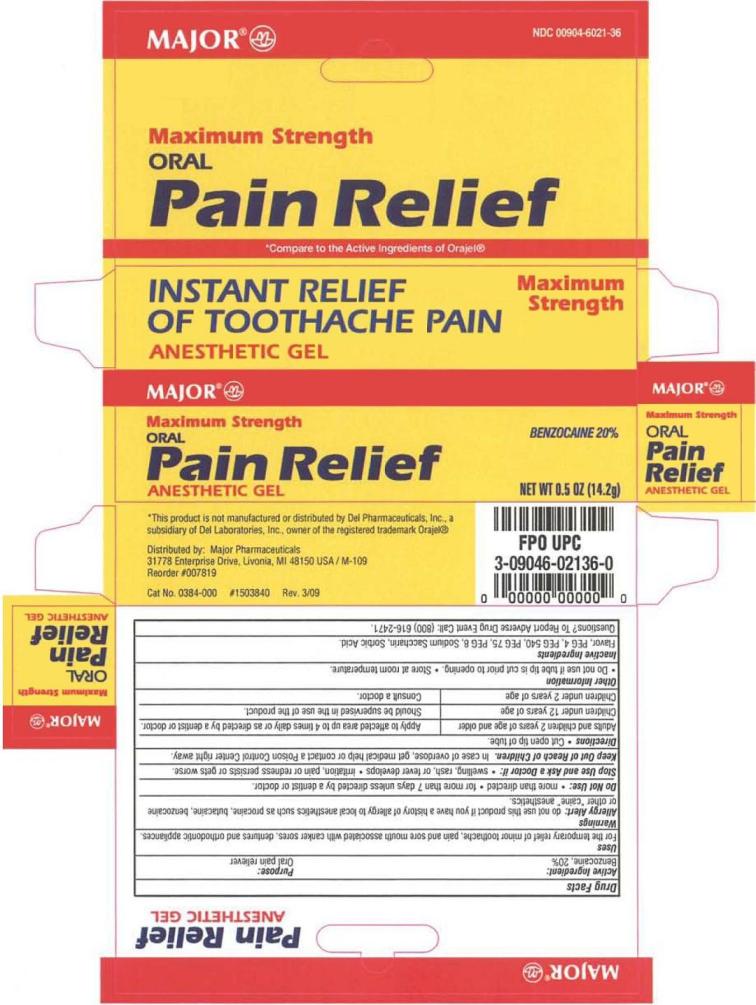
ORAL PAIN RELIEF ANESTHETIC ANESTHETIC- benzocaine gel
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
For the temporary relief of minor toothache, pain and sore mouth associated with canker sores, dentures and orthodontic appliances.
Allergy Alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Do not use
Do Not Use
- •
- more than directed
- •
- for more than 7 days unless directed by a dentist or doctor.
Stop use
Stop use and ask a Doctor if:
- •
- swelling, rash, or fever develops
- •
- irritation, pain or redness persists or gets worse
Keep out of reach of children
Keep Out of Reach of Children. In case of overdose, get medical help or contact a Poison Control Center right away.
| ORAL PAIN RELIEF ANESTHETIC
ANESTHETIC
benzocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |