LAXATIVE PILLS- sennosides tablet
H E B
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
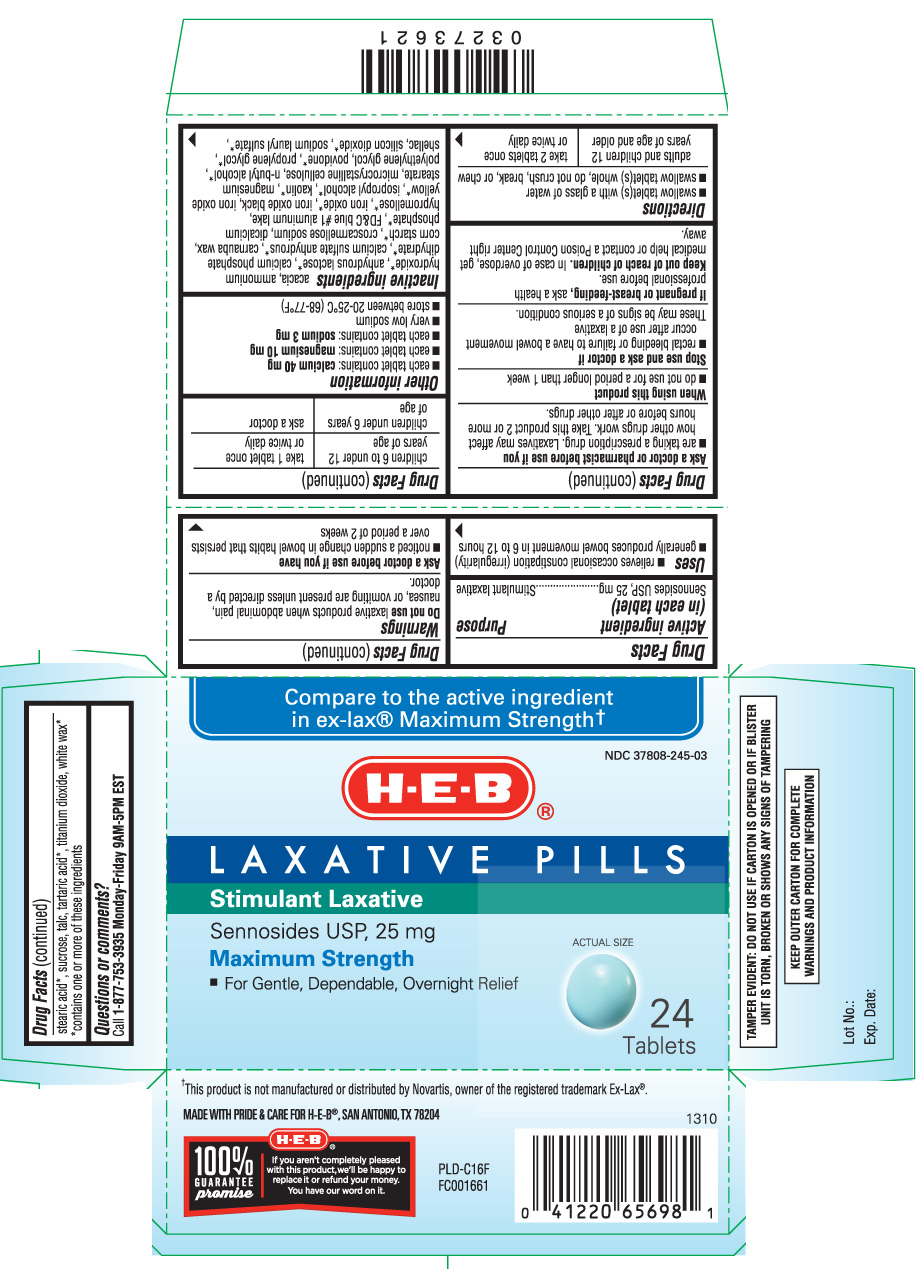
Drug Facts
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use if you have
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor or pharmacist before use if you
- are taking a prescription drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
Directions
- swallow tablet(s) with a glass of water
- swallow tablet(s) whole, do not crush, break, or chew
| adults and children 12 years of age and older | take 2 tablets once or twice daily |
| children 6 to under 12 years of age | take 1 tablet once or twice daily |
| children under 6 years of age | ask a doctor |
Other information
- each tablet contains: calcium 40 mg
- each tablet contains: magnesium 10 mg
- each tablet contains: sodium 3 mg
- very low sodium
- store between 20-25ºC (68-77ºF)
Inactive ingredients
acacia, ammonium hydroxide*, anhydrous lactose*, calcium phosphate dihydrate*, calcium sulfate anhydrous*, carnauba wax, corn starch*, croscarmellose sodium, dicalcium phosphate*, FD&C blue #1 aluminum lake, hypromellose*, iron oxide*, iron oxide black, iron oxide yellow*, isopropyl alcohol*, kaolin*, magnesium stearate, microcrystalline cellulose, n-butyl alcohol*, polyethylene glycol, povidone*, propylene glycol*, shellac, silicon dioxide*, sodium lauryl sulfate*, stearic acid*, sucrose, talc, tartaric acid*, titanium dioxide, white wax*
*contains one or more of these ingredients
Principal Display Panel
Compare to the active ingredient in ex-lax® Maximum Strength†
Laxative Pills
Stimulant Laxative
Sennosides USP, 25 mg
Maximum Strength
- For gentle, dependable, overnight relief
Tablets
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
†This product is not manufactured or distributed by Novartis, owner of the regsitered trademark Ex-Lax®
Made with pride and care for HEB®, San Antonio, TX 78204
| LAXATIVE PILLS
sennosides tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - H E B (007924756) |