TOKUHON-A EXTERNAL PAIN RELIEVING MEDICATED- camphor (natural), menthol, methyl salicylate patch
Tokuhon Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
TOKUHON-A External Pain Relieving Medicated Patch
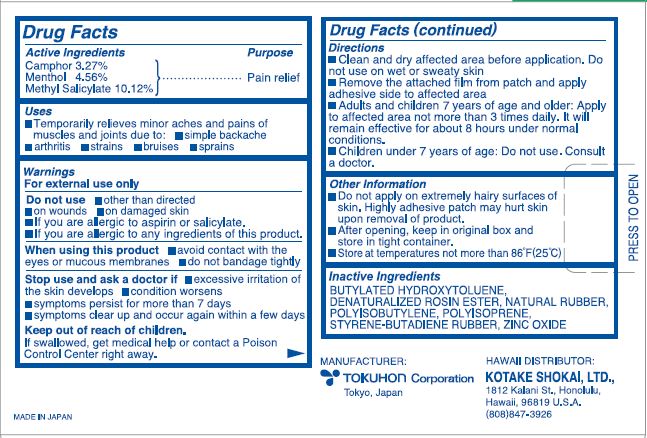
Uses
Temporarily relief of minor aches and pains of muscles and joints due to:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Do not use
- otherwise than directed
- on wounds
- on damaged skin
- If you are allergic to aspirin or salicylate.
- If you are allergic to any ingredients of this product.
Directions
- Clean and dry the affected area before application. Do not use on wet or sweaty skin.
- Remove the attached film from patch and apply adhesive side to affected area.
- Adults and children 7 years of age and older: Apply to affected area not more than 3 times daily. It will remain effective for about 8 hours under normal conditions.
- Children under 7 years of age: Do not use. Consult a doctor.
Other Information
- Do not apply on extremely hairy surfaces of skin. Highly adhesive patch may hurt skin upon removal of product.
- After opening, keep in original box and store in tight container.
- Store at temperatures not more than 86 oF(25 oC)
Inactive Ingredients
Butylated Hydroxytoluene, Denaturalized Rosin Ester, Natural Rubber, Polyisobutylene, Polyisoprene, Styrene-butadiene Rubber, Zinc Oxide
MANUFACTURER:
MANUFACTURER:
TOKUHON CORPORATION
Tokyo, Japan
HAWAII DISTRIBUTOR:
KOTAKE SHOKAI, LTD.,
1812 Kalani St., Honolulu,
Hawaii, 96819 U.S.A.
(808)847-3926
MADE IN JAPAN
| TOKUHON-A EXTERNAL PAIN RELIEVING MEDICATED
camphor (natural), menthol, methyl salicylate patch |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Tokuhon Corporation (715715442) |
| Registrant - Tokuhon Corporation (715715442) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Tokuhon Corporation | 715715442 | manufacture(48407-0070) | |