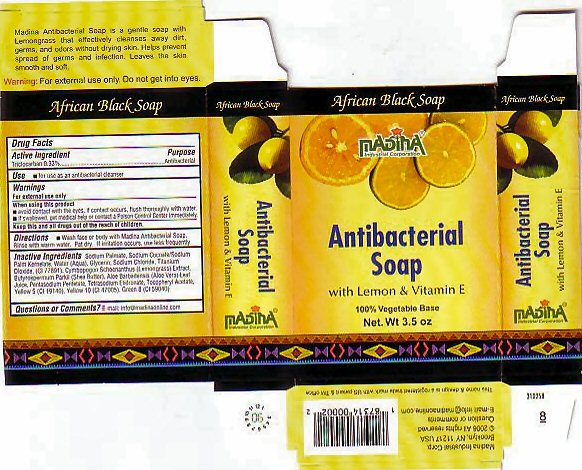
MADINA AFRICAN BLACK- triclocarban soap
Bradford Soap Works, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
USE
FOR USE AS AN ANTIBACTERIAL CLEANSER
DIRECTIONS
- WASH FACE OR BODY WITH MADINA ANTIBACTERIAL SOAP. RINSE WITH WARM WATER. PAT DRY. IF IRRITATION OCCURS, USE LES FREQUENTLY.
INACTIVE INGREDIENTS
SODIUM PALMATE, SODIUM COCOATE/SODIUM PALM KERNELATE, WATER (AQUA), GLYCERIN, SODIUM CHLORIDE, TITANIUM DIOXIDE, (CI 77891), CYMBOPOGON SCHOENANTHUS (LEMONGRASS) EXTRACT, BUTYROSPERMUM PARKII (SHEA BUTTER), ALOE BARBADENSUS (ALOE VERA) LEAF JUICE, PENTASODIUM PENTETATE, TETRASODIUM ETIDRONATE, TOCOPHERYL ACETATE, YELLOW 5 (CI 19140), YELLOW 10 (CI 47005), GREEN 8 (CI 59040)
Africian Black Soap
Africian Black Soap
Madina
Antibacterial Soap
with Lemon & VitaminE
100% Vegetable Base
Net. Wt. 3.5 oz
Madina Antibacterial Soap is a gentle soap with Lemongrass that effectively cleanses away dirt, germs, and orors without drying skin. Helps preveny spread of germs and infection. Leaves the skin smooth and soft.
Warning: For external use only. Do not get into eyes.
Madina Industrial Corp.
Brooklyn, NY 11217 USA
© 2006 All rights reserved
Questions or comments
E-mail: info@madinaonline.com
This name and design is a registered trade mark with the US patent & TM office
| MADINA AFRICAN BLACK
triclocarban soap |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Bradford Soap Works, Inc. (001201045) |
| Registrant - Bradford Soap Works, Inc. (001201045) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc. | 001201045 | manufacture(11118-1104) , label(11118-1104) | |