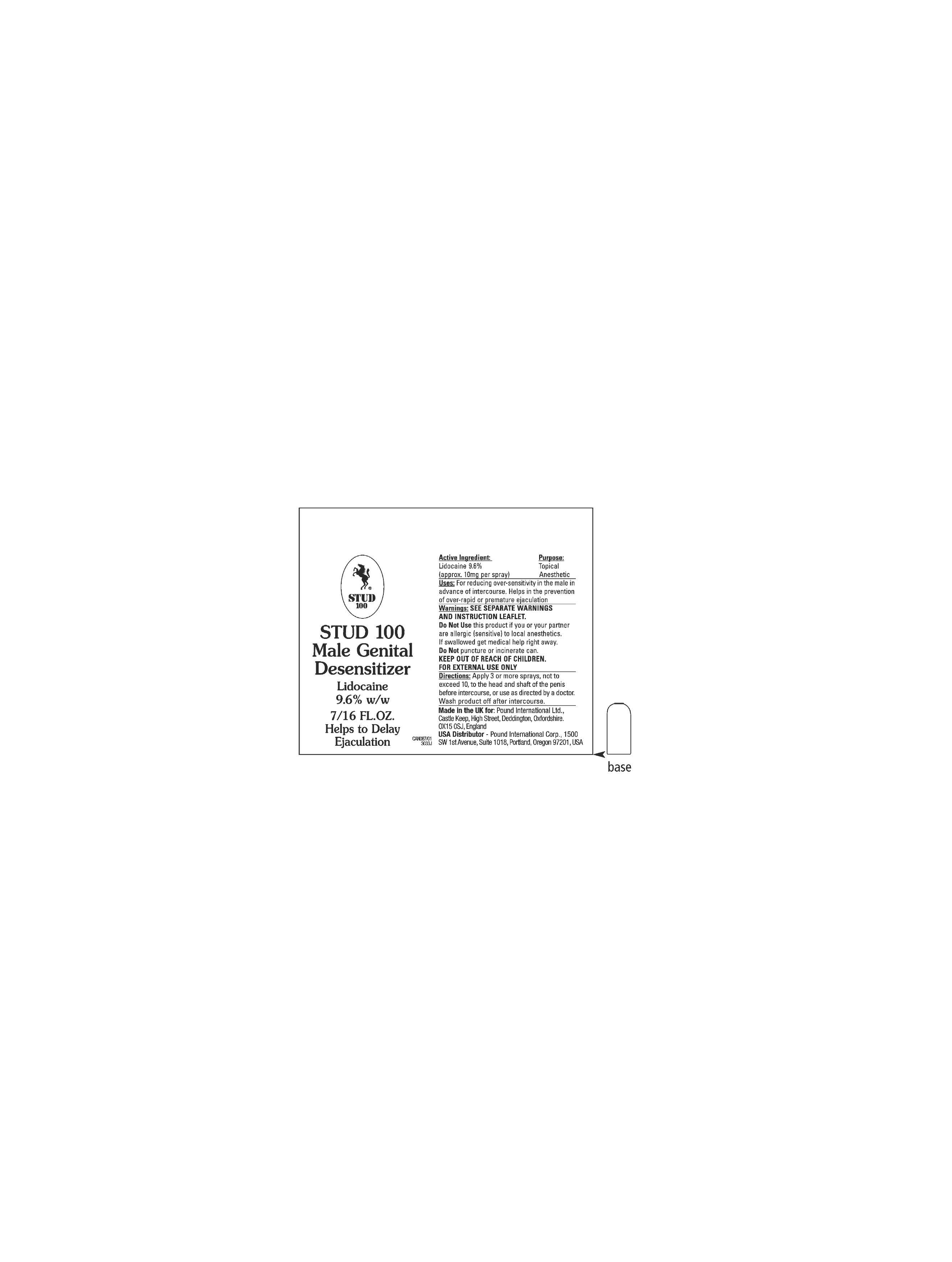
Label: MALE GENITAL DESENSITIZER STUD 100- lidocane spray, metered
- NDC Code(s): 57707-050-01, 57707-050-02
- Packager: Pound International Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Stop and consult a doctor
if you or your partner develop a rash or irritation, such as burning or itching
if you do feel unwell or have any unpleasant effects after using the spray
If this product, used as directed, does not provide relief. Premature ejaculation may be due to a condition requiring medical supervision
-
WARNINGS
Warnings
For external use only
Allergy alert: do not use this product if you or your partner are allergic (sensitive) to local Anesthetics.
Do not use - on broken or inflamed skin -if your partner is pregnant
Ask a doctor before use if you have, or ever had, liver or kidney problems
Ask a doctor or pharmacist before use if you are already taking prescribed drugs
when using this product
- do not get into eyes
- do not inhale
- do not exceed 24 sprays in 24 hours
-
DOSAGE & ADMINISTRATION
Directions
-apply 3 or more sprays, not to exceed 10, to the head and shaft of the penis before intercourse, or as directed by a doctor
-wash product after intercourse
-correct quantity and time of application will be determined by individual requirements and you should always use the minimum effective quantity
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MALE GENITAL DESENSITIZER STUD 100
lidocane spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57707-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 9.6 mg in 100 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57707-050-02 1 in 1 CARTON 03/13/2013 1 NDC:57707-050-01 12.5 mL in 1 CAN; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/13/2013 Labeler - Pound International Corp (802685065)