CHILDRENS CHEWABLE ACETAMINOPHEN- acetaminophen tablet, chewable
Gemini Pharmaceuticals, Inc. dba Plus Pharma
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Children's Chewable Acetaminophen
Uses
Temporarily relieves minor aches and pain due to:
■ The common cold ■ Flu ■ Headache ■ Sore throat
■ Toothache
Temporarily reduces fever.
Warnings
Liver Warning: This Product contains acetaminophen. Severe liver damage may occur if your child takes
■ more than 5 doses in 24 hours, which is the maximum daily amount
■ with other drugs containing acetaminophen.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
- If your child is allergic to acetaminophen or any of the inactive ingredients in this products.
Directions
- This products does not contain directions or complete warning for adult use.
- Find right dose on the chart below. If possible, use weight to dose; otherwise, use age.
- Chew before swallowing
- If needed, repeat dose every 4 hours while symptoms lasts
- Do not give more than 5 times in 24 hours
- Do not give for more than 5 days unless directed by a doctor.
| WEIGHT | AGE | TABLETS |
| Under 24 lbs | Under 2 years | Ask a doctor |
| 24-35 lbs | 2-3 years | 2 tablets |
| 36-47 lbs | 4-5 years | 3 tablets |
| 48-59 lbs | 6-8 years | 4 tablets |
| 60-71 lbs | 9-10 years | 5 tablets |
| 72-95 lbs | 11 years | 6 tablets |
Other Information
- Phenylketonurics: Contain phenylalanine 14 mg per tablet
- Store at room temperature in a dry place
Inactive Ingredients
Aspartame, Colloidal Silica, D&C Red # 27 Lake, Hypromellose, Magnesium Stearate, Mannitol, Natural & Artificial Berry Flavors, Povidone, Pregelatinized Starch, Sodium Chloride, Sodium Starch Glycolate, Sorbitol, Stearic Acid and Sucralose.
Questions?
If you have any questions or comments, or to report an adverse drug event, please contact (800) 795-9775.
Principal Display Panel
See New Warning Information
PlusPHARMA
TM
Children's Chewable
ACETAMINOPHEN
PAIN RELIEVER/FEVER REDUCER
CONTAINS NO ASPIRIN
*Compare the active ingredients to Children Chewable Tylenol ®
30 Chewable Tablet
■ 80 mg Each
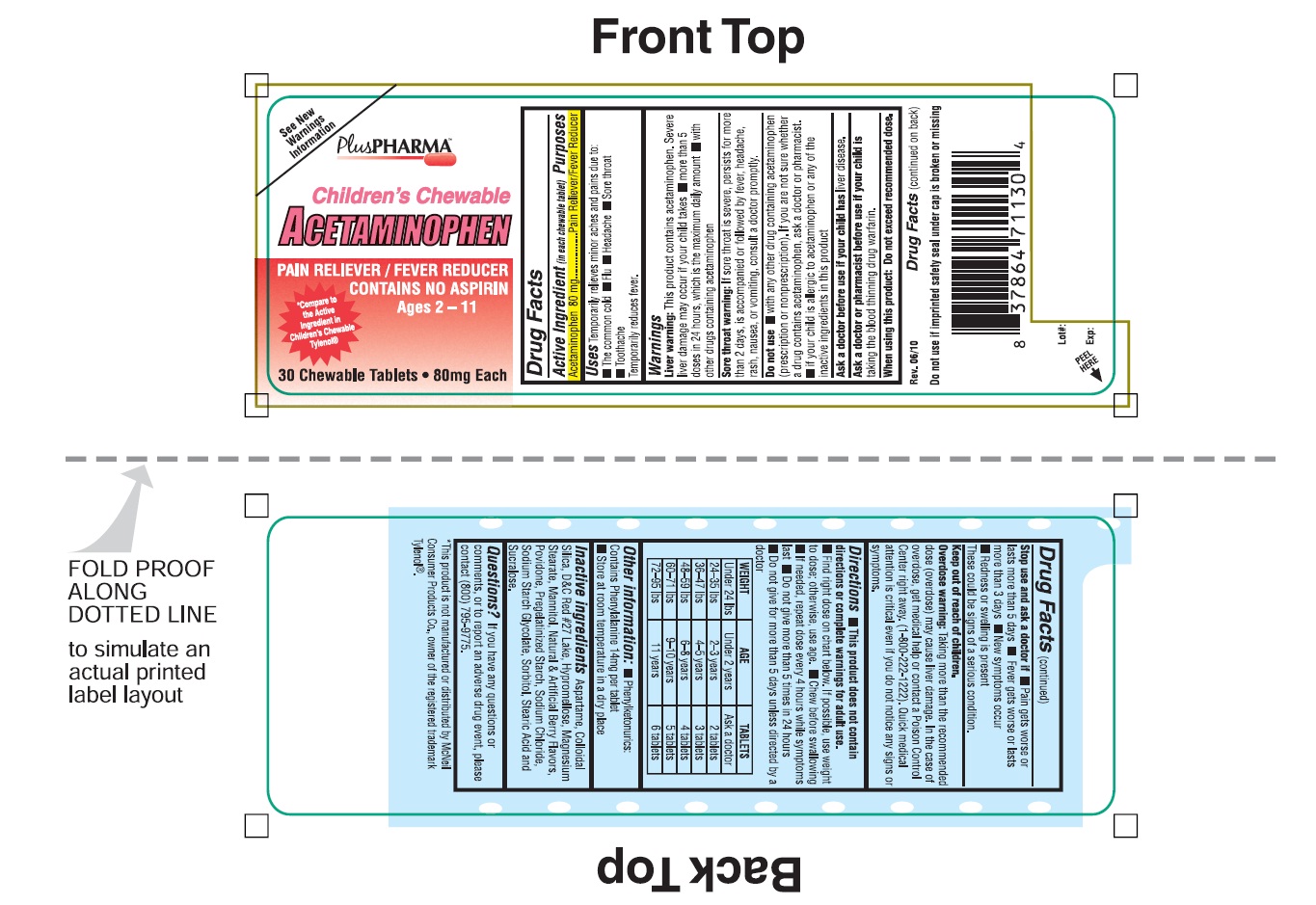
Rev. 06/10
Do not use if imprinted seal under cap is broken or missing.
| CHILDRENS CHEWABLE ACETAMINOPHEN
acetaminophen tablet, chewable |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Gemini Pharmaceuticals, Inc. dba Plus Pharma (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gemini Pharmaceuticals, Inc. dba Plus Pharma | 055942270 | manufacture(51645-711) | |