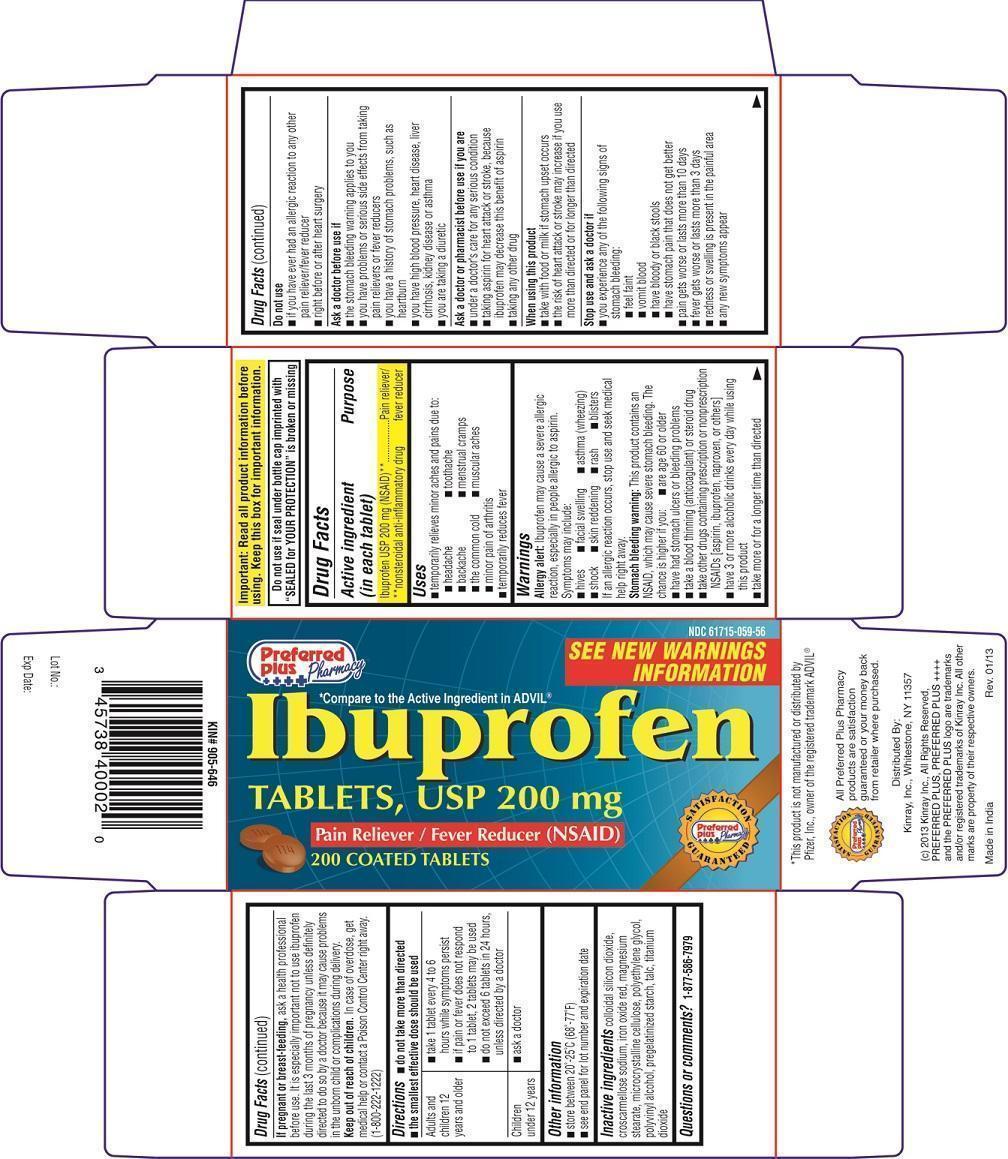
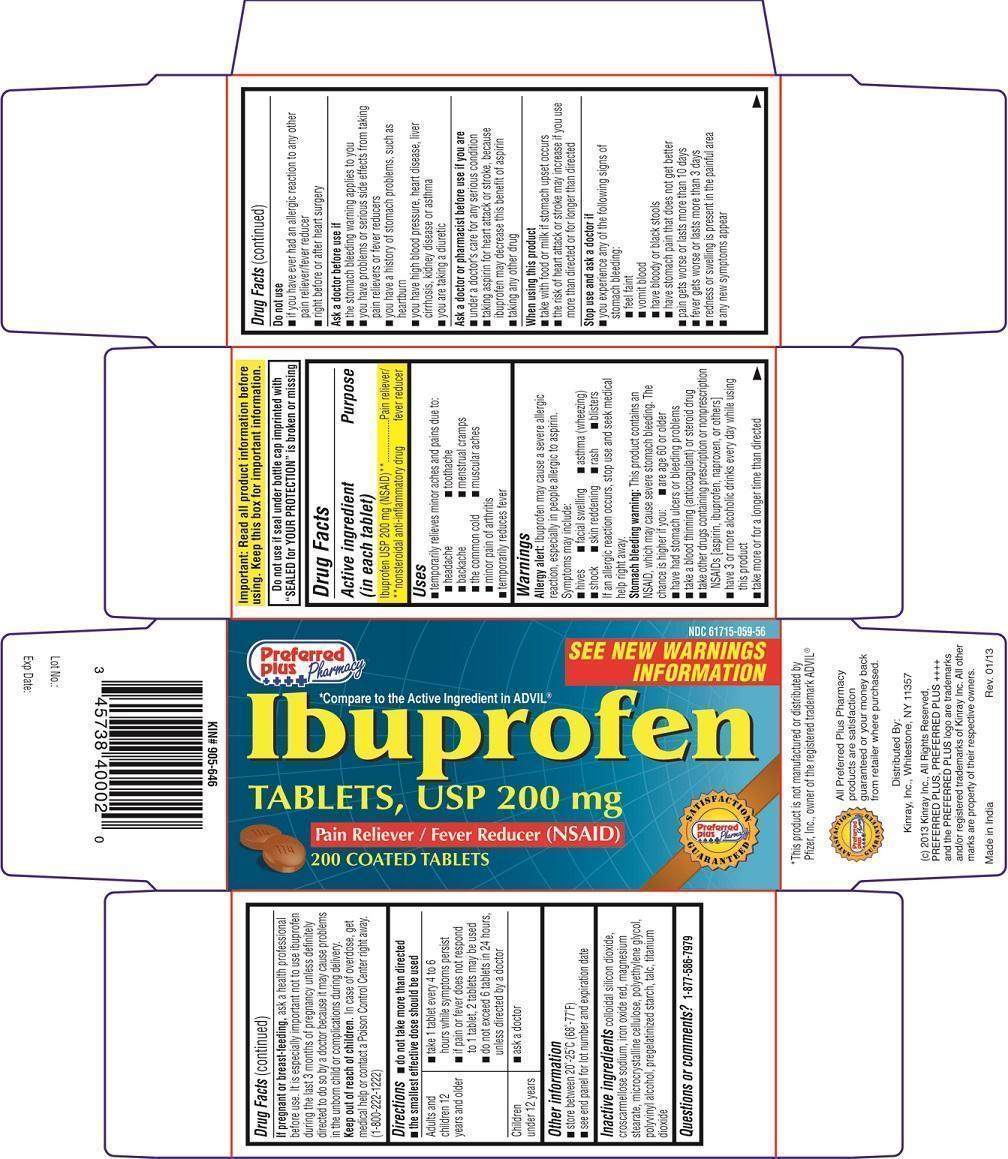
Label: IBUPROFEN- ibuprofen tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 61715-059-56 - Packager: Kinray
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks everyday while using this product
- take more or for a longer time than directed
- Do not use
-
Ask a doctor before use if
- the stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease or asthma
- you are taking a diuretic
- Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
- If pregnant or breast-feeding
- Keep out of reach of children
-
Directions
- do not take more than directed
- the smallest effective dose should be used
Adults and children 12 years and older - take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
Children under 12 years - ask a doctor
- Other Information
- Inactive Ingredients:
- Questions or comments? 1-877-586-7979
-
PRINCIPAL DISPLAY PANEL - 200 mg TABLET CARTON
Preferred Plus Pharmacy
NDC 61715-059-56
SEE NEW WARNINGS INFORMATION
*Compare to the Active Ingredient in ADVIL®
Ibuprofen TABLETS, USP 200 mg
Pain Reliever/Fever Reducer (NSAID)
200 COATED TABLETS
Distributed By:
Kinray, Inc., Whitestone, NY 11357
(c) 2013 Kinray Inc., All Rights Reserved, PREFERRED PLUS, PREFERRED PLUS ++++ and the PREFERRED PLUS logo are trademarks and/or registered trademarks of Kinray Inc. All other marks are property of their respective owners.
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61715-059 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code 114 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61715-059-56 1 in 1 CARTON 1 200 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091239 07/25/2011 Labeler - Kinray (012574513) Registrant - Aphena Pharma Solutions - Kentucky, LLC (557054835) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Kentucky, LLC 557054835 repack(61715-059) Establishment Name Address ID/FEI Business Operations Marksans Pharma Limited 925822975 manufacture(61715-059)