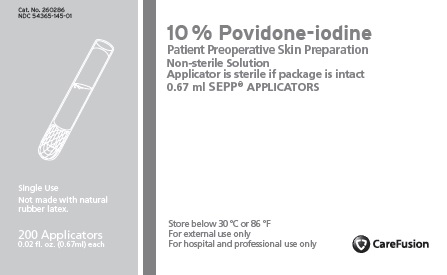
PVP-IODINE SEPP- povidone iodine solution
CareFusion 213 LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PVP-Iodine 10% SEPP
Uses
- Patient preoperative skin preparation. Helps to reduce bacteria that potentially can cause skin infection.
Warnings
For external use only.
Directions
- remove the applicator wrapper. Do not touch the applicator tip.
- with applicator tip facing downward, pinch the barrel of the applicator ONCE to release the antiseptic.
- press the applicator tip against the patient’s skin. With gentle back and forth actions apply solution to the treatment area.
- the area covered should be allowed to dry naturally.
- discard after single use.
| PVP-IODINE SEPP
povidone iodine solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - CareFusion 213 LLC (826496312) |
| Registrant - CareFusion 2200, Inc (832696038) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CareFusion 213 LLC | 826496312 | label(54365-145) , manufacture(54365-145) , pack(54365-145) | |
Revised: 12/2017
Document Id: 5f99d233-545f-265b-e053-2a91aa0a6d48
Set id: cfecd7de-fa13-493f-9997-8bbea50c671f
Version: 5
Effective Time: 20171207
CareFusion 213 LLC