Label: KIDS-EEZE- diphenhydramine hydrochloride tablet, orally disintegrating
-
Contains inactivated NDC Code(s)
NDC Code(s): 61941-1005-1, 61941-1005-6 - Packager: ProPhase Labs, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each soft chew)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
-
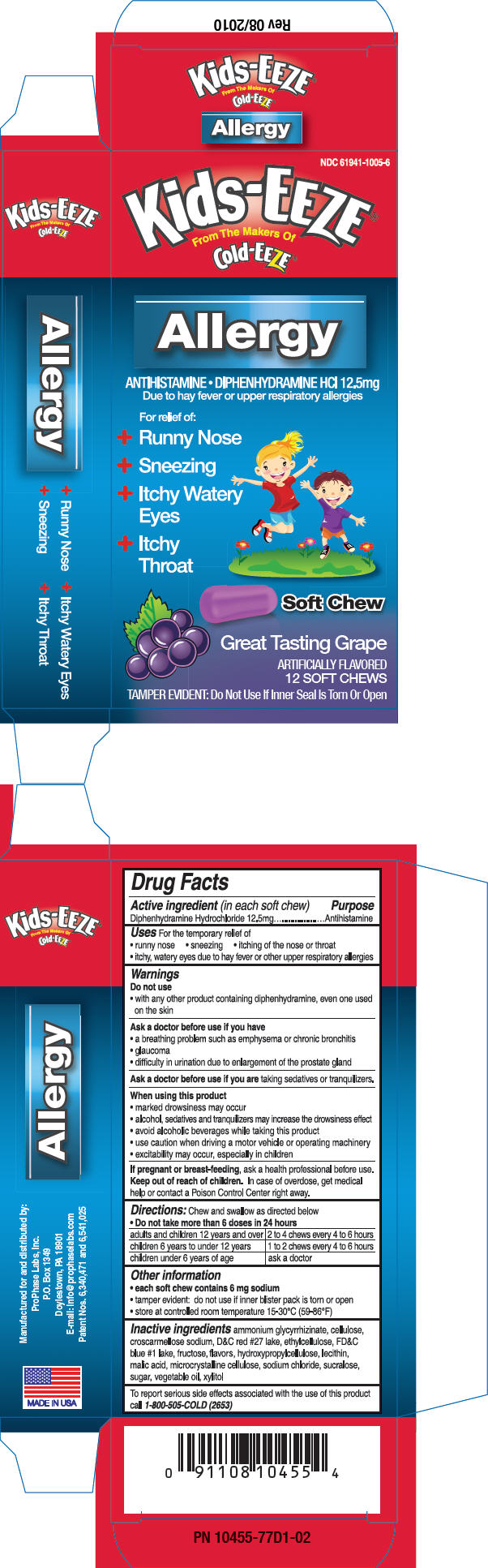
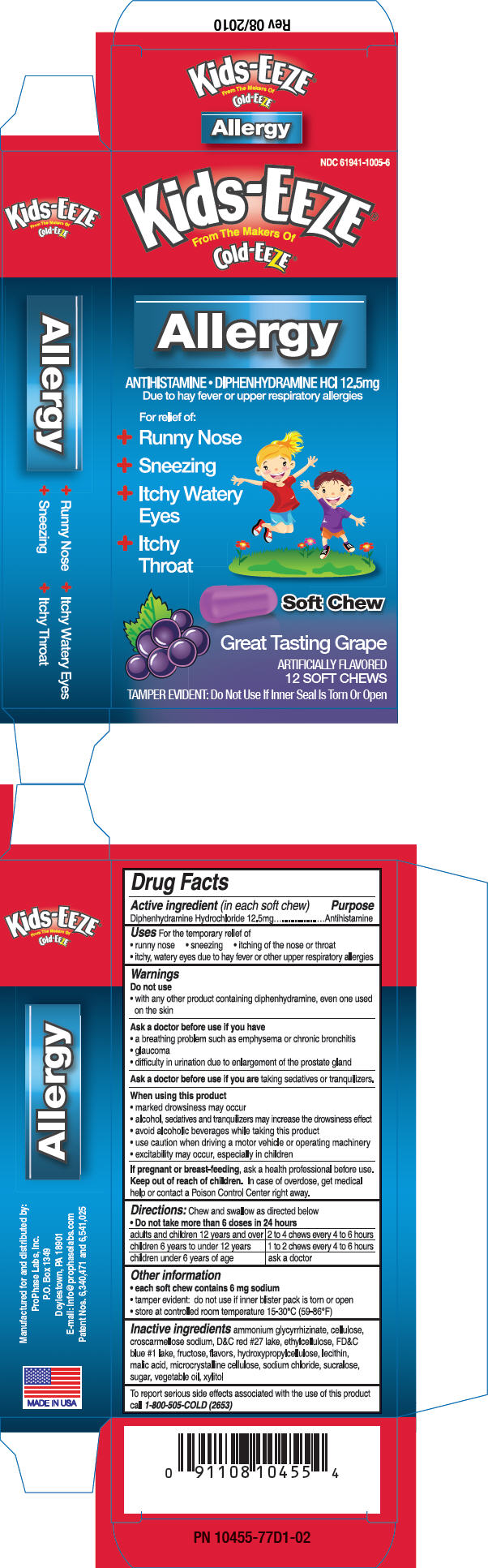
PRINCIPAL DISPLAY PANEL - 12.5 mg Package
NDC 61941-1005-6
Kids-EEZE®
From The Makers Of
Cold-EEZE®Allergy
ANTIHISTAMINE • DIPHENHYDRAMINE HCl 12.5mg
Due to hay fever or upper respiratory allergiesFor relief of:
- +
- Runny Nose
- +
- Sneezing
- +
- Itchy Watery
Eyes - +
- Itchy
Throat
Soft Chew
Great Tasting Grape
ARTIFICIALLY FLAVORED
12 SOFT CHEWSTAMPER EVIDENT: Do Not Use If Inner Seal Is Torn Or Open
-
INGREDIENTS AND APPEARANCE
KIDS-EEZE ALLERGY
diphenhydramine hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61941-1005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diphenhydramine Hydrochloride (UNII: TC2D6JAD40) (Diphenhydramine - UNII:8GTS82S83M) Diphenhydramine Hydrochloride 12.5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYLCELLULOSE (4 MPA.S) (UNII: KC5472WRJK) FRUCTOSE (UNII: 6YSS42VSEV) MALIC ACID (UNII: 817L1N4CKP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) Product Characteristics Color PURPLE Score no score Shape OVAL Size 17mm Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61941-1005-1 72 in 1 CASE 1 NDC:61941-1005-6 12 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 09/01/2010 Labeler - ProPhase Labs, Inc. (620557298) Establishment Name Address ID/FEI Business Operations ProPhase Labs, Inc. 620557298 LABEL, ANALYSIS Establishment Name Address ID/FEI Business Operations Pharmaloz Manufacturing, Inc. 067101998 MANUFACTURE, ANALYSIS, PACK, REPACK