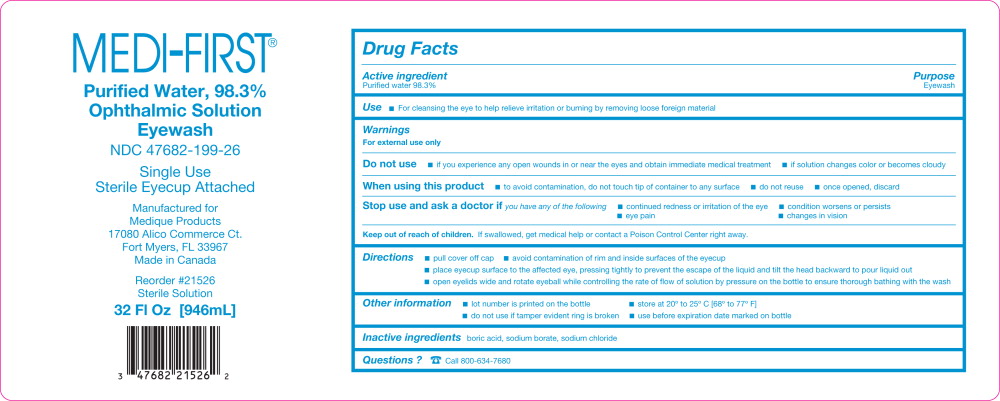
MEDI-FIRST EYEWASH- water solution
Unifirst First Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
Directions
- pull cover off cap
- avoid contamination of the rim and inside surface of the eyecup
- place eyecup surface to the affected eye, pressing tightly to prevent the escape of the liquid and tilt the head backward to pour liquid out
- open eyelids wide and rotate eyeball while controlling the rate of flow of solution by pressing the bottle to ensure thorough bathing with the wash
Other information
- lot number is printed on the bottle
- store at 20º to 25º C [68º to 77º F]
- do not use if tamper evident ring is broken
- use before expiration date marked on bottle
Medi-First Niagara Eyewash Label
Medi-First®
Purified Water, 98.3%
Ophthalmic Solution
Eyewash
NDC 47682-199-26
Single Use
Sterile Eyecup Attached
Manufactured for
Medique Products
17080 Alico Commerce Ct.
Fort Myers, FL 33967
Made in Canada
Reorder #21526
Sterile Solution
32 Fl Oz [946 mL]
3 47682 21526 2
| MEDI-FIRST
EYEWASH
water solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Unifirst First Aid Corporation (832947092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Niagara Pharmaceuticals Inc. | 205477792 | manufacture(47682-199) | |
Revised: 5/2018
Document Id: 6b2669ca-5222-291b-e053-2991aa0a5d60
Set id: cf1786ae-2f92-4928-b399-aa83f659ff75
Version: 3
Effective Time: 20180501
Unifirst First Aid Corporation