Label: ARNICARE- arnica montana gel
-
NDC Code(s):
0220-9000-54,
0220-9000-59,
0220-9000-65,
0220-9000-66, view more0220-9000-84, 0220-9000-86, 0220-9000-94
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
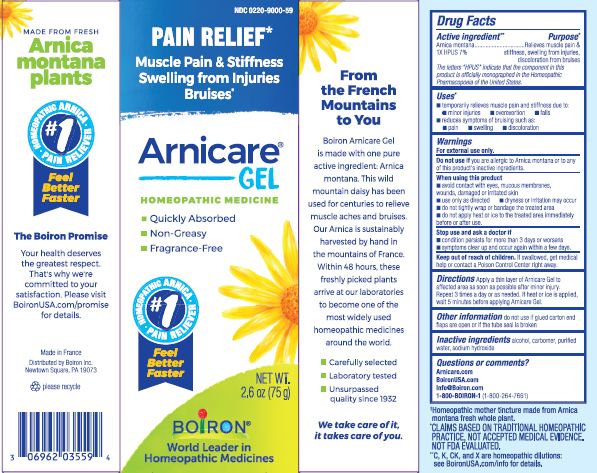
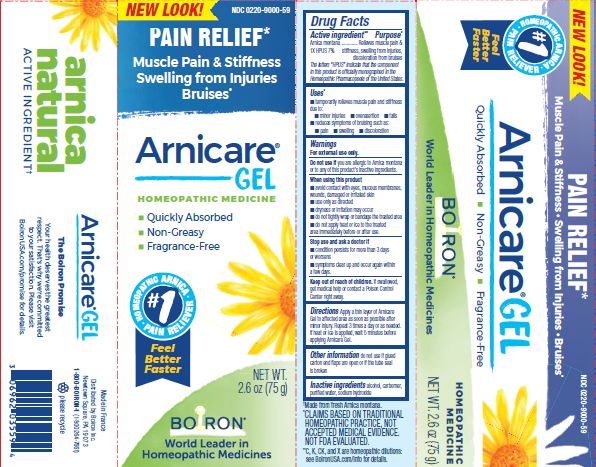
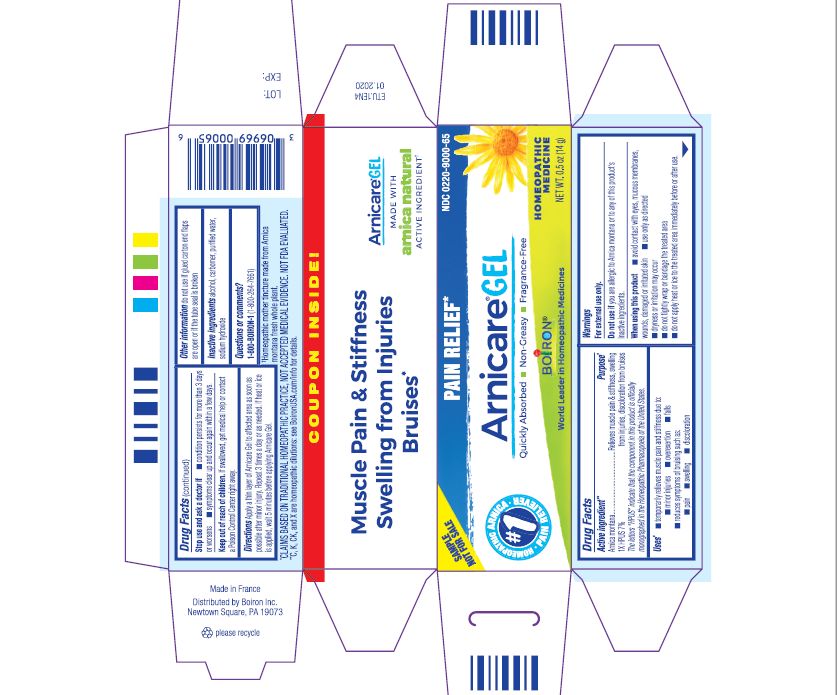
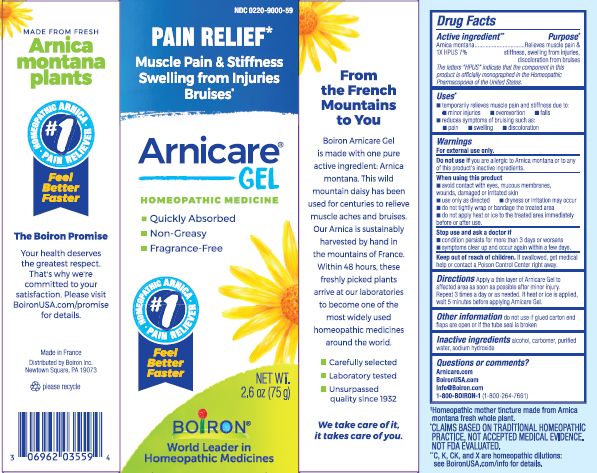
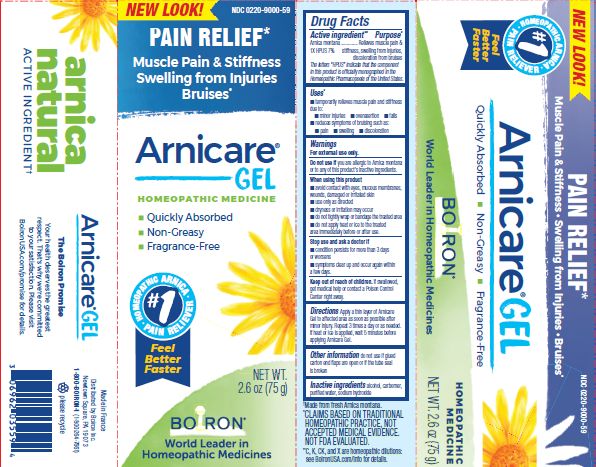
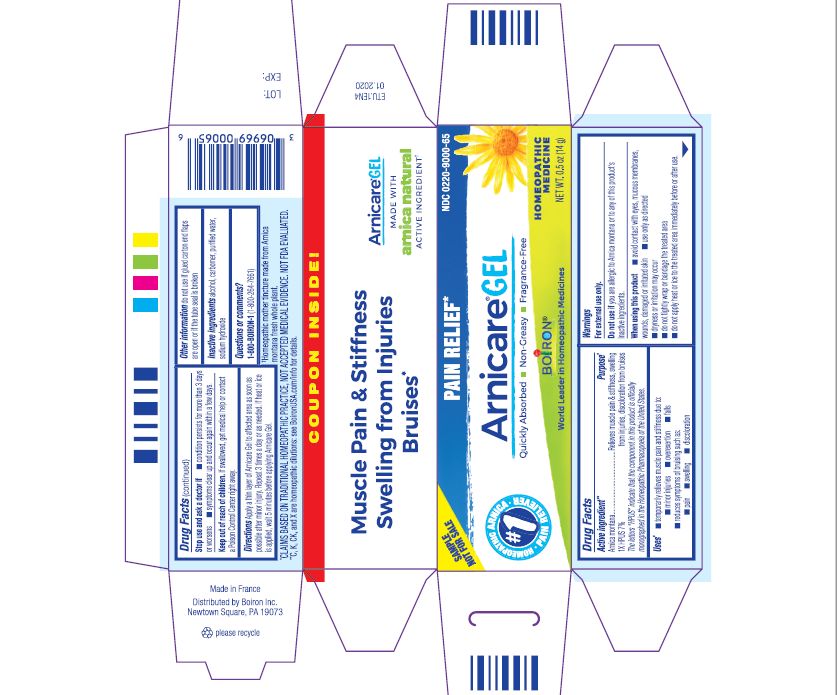
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
Do not use if you are allergic to Arnica montana or to any of this product's inactive ingredients.
When using this product
avoid contact with eyes, mucous membranes, wounds, damaged or irritated skin
use only as directed
dryness or irritation may occur
do not tightly wrap or bandage the treated area
do not apply heat or ice to treated area immediately before or after use
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
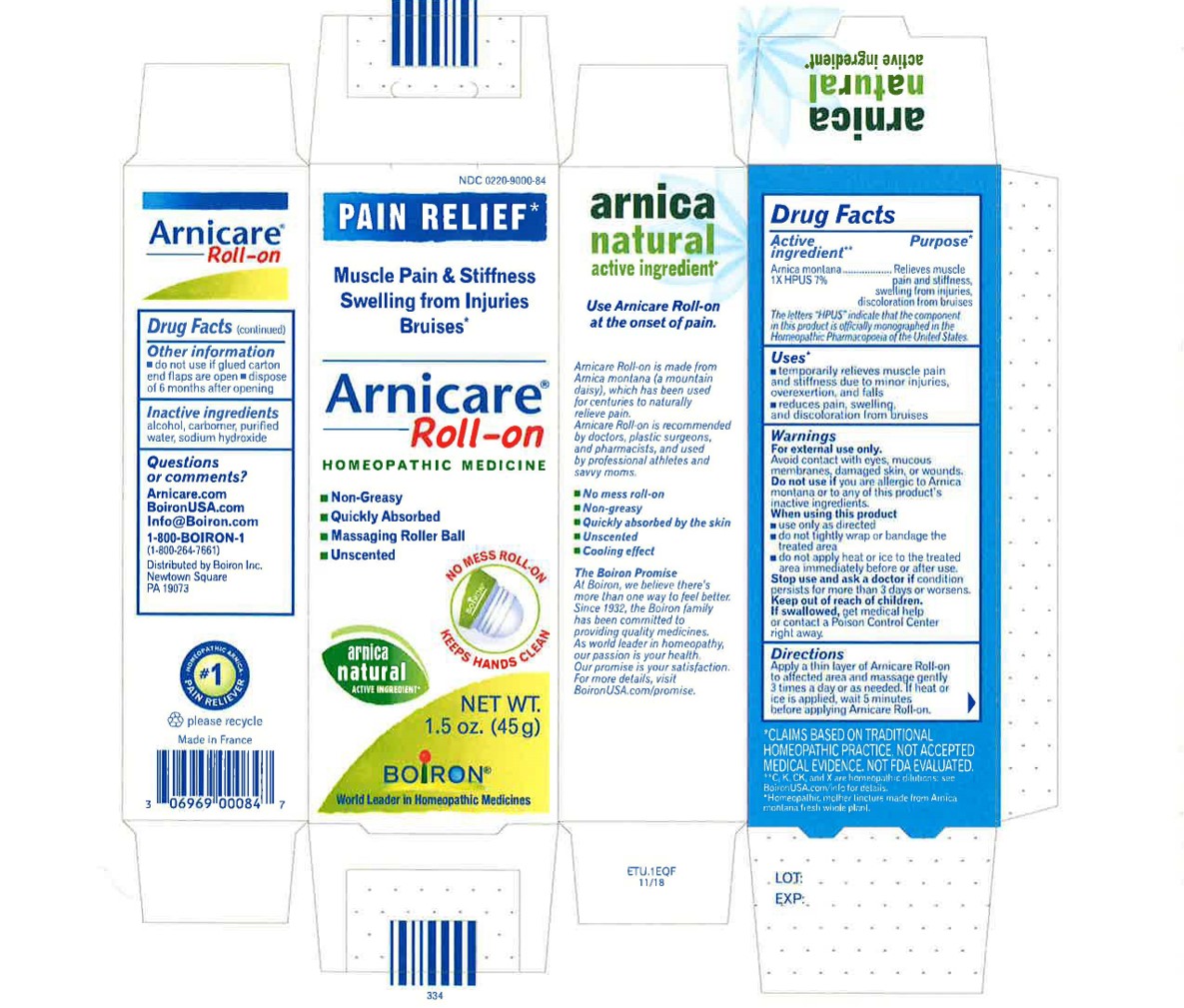
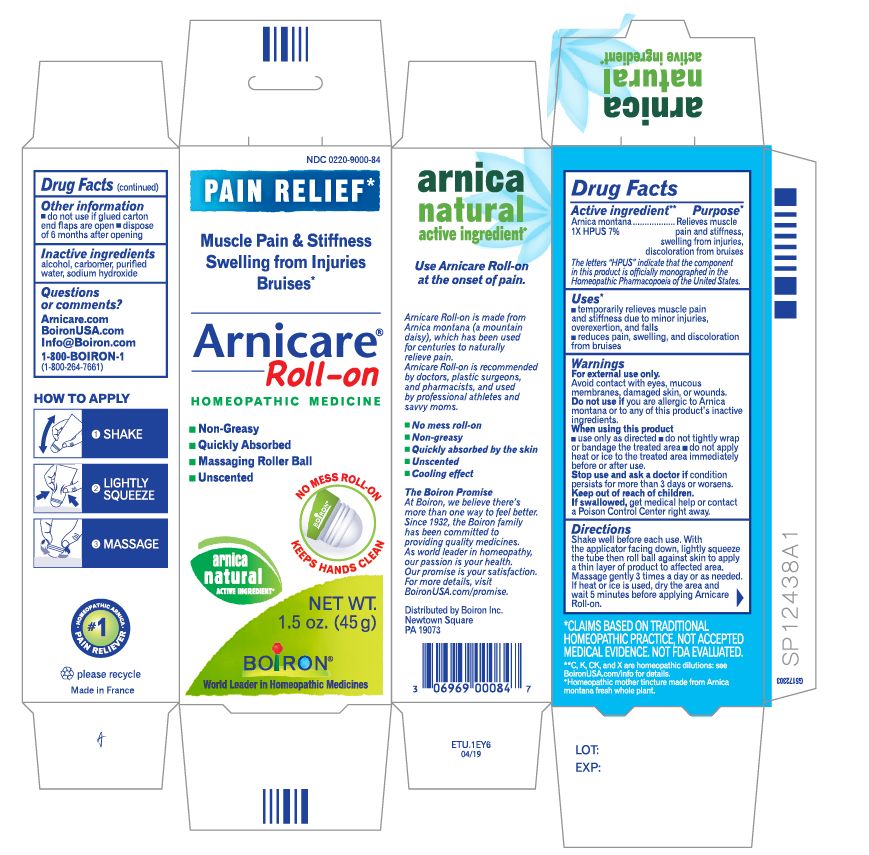
Directions
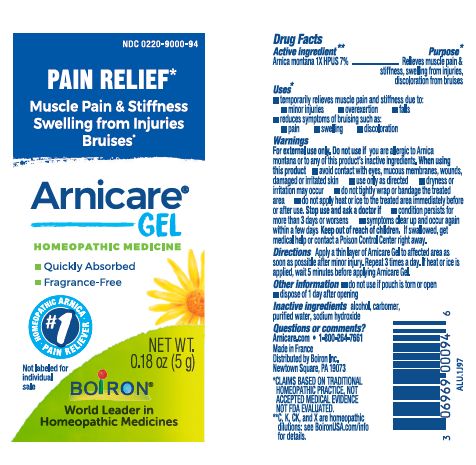
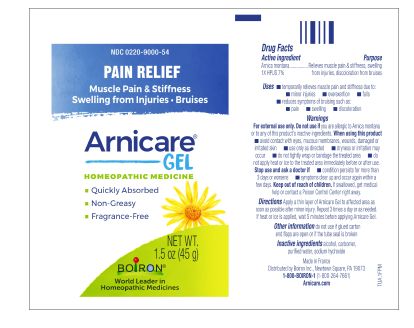
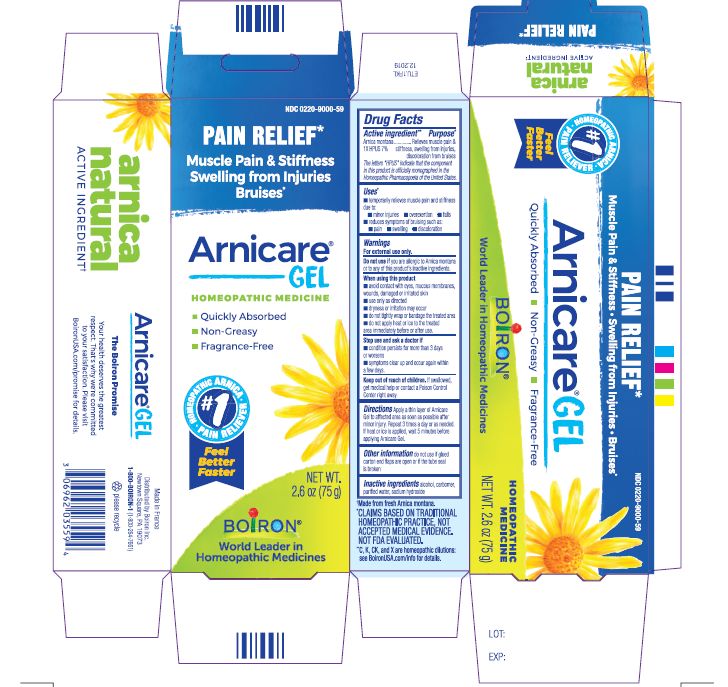
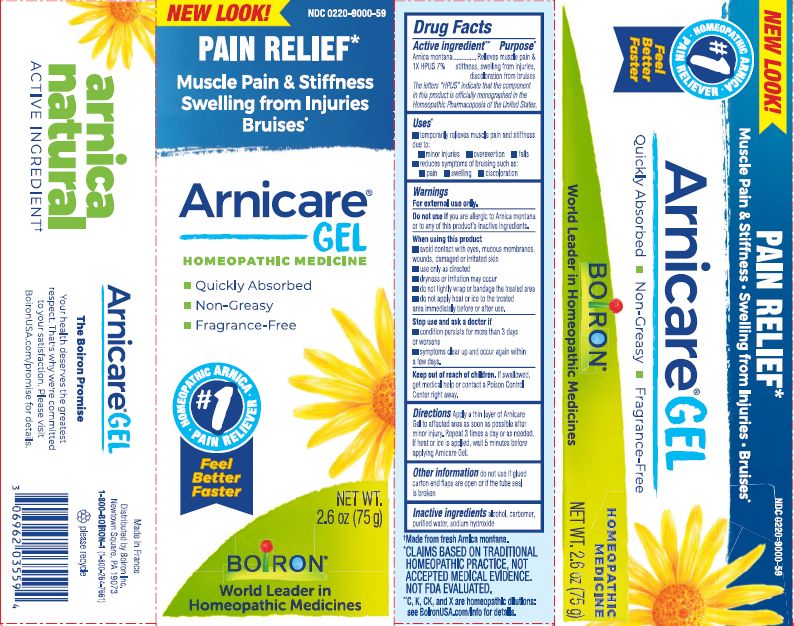
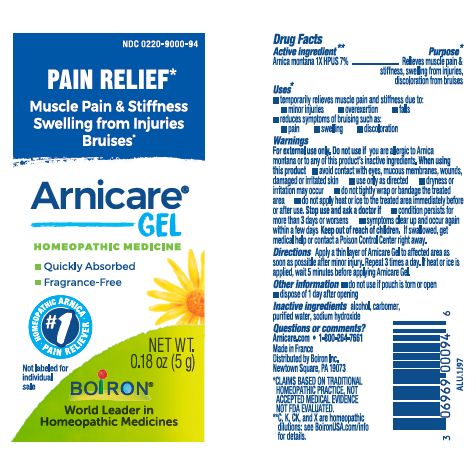
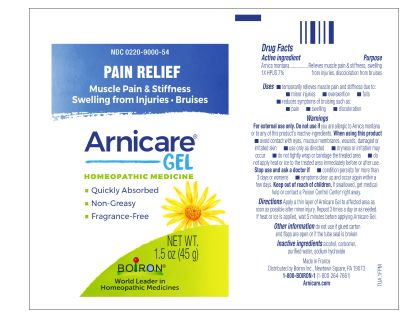
Apply a thin layer of Arnicare Gel to the affected area as soon as possible after minor injury. Repeat 3 times a day or as needed. If heat or ice is applied, wait 5 minutes before applying Arnicare Gel.
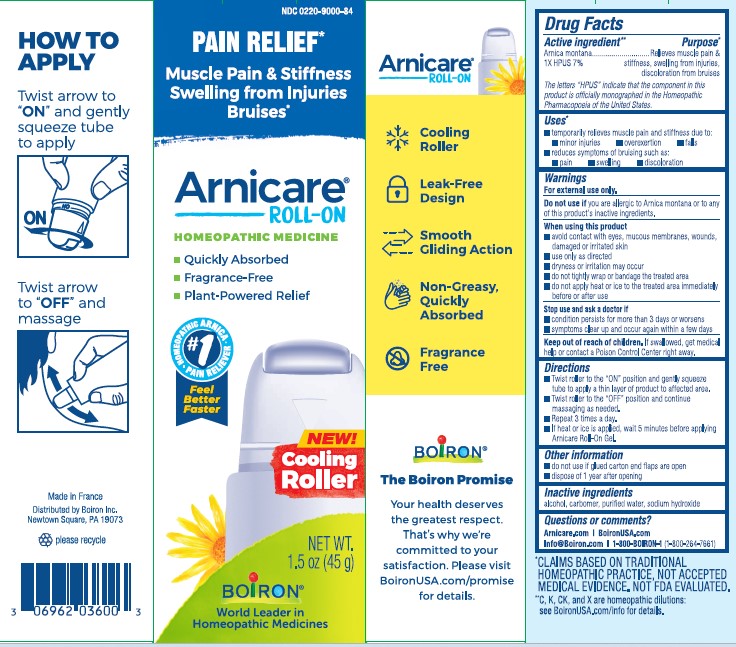
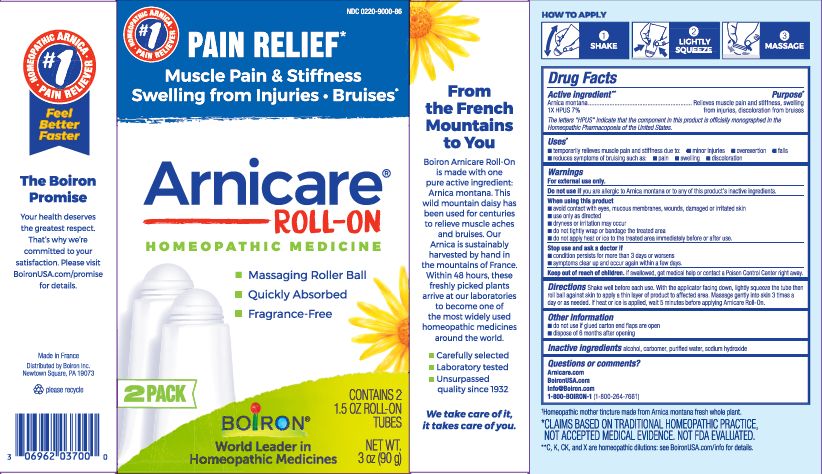
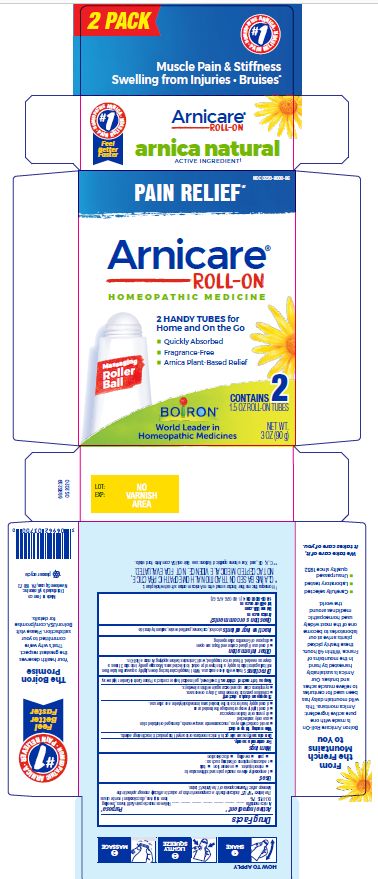
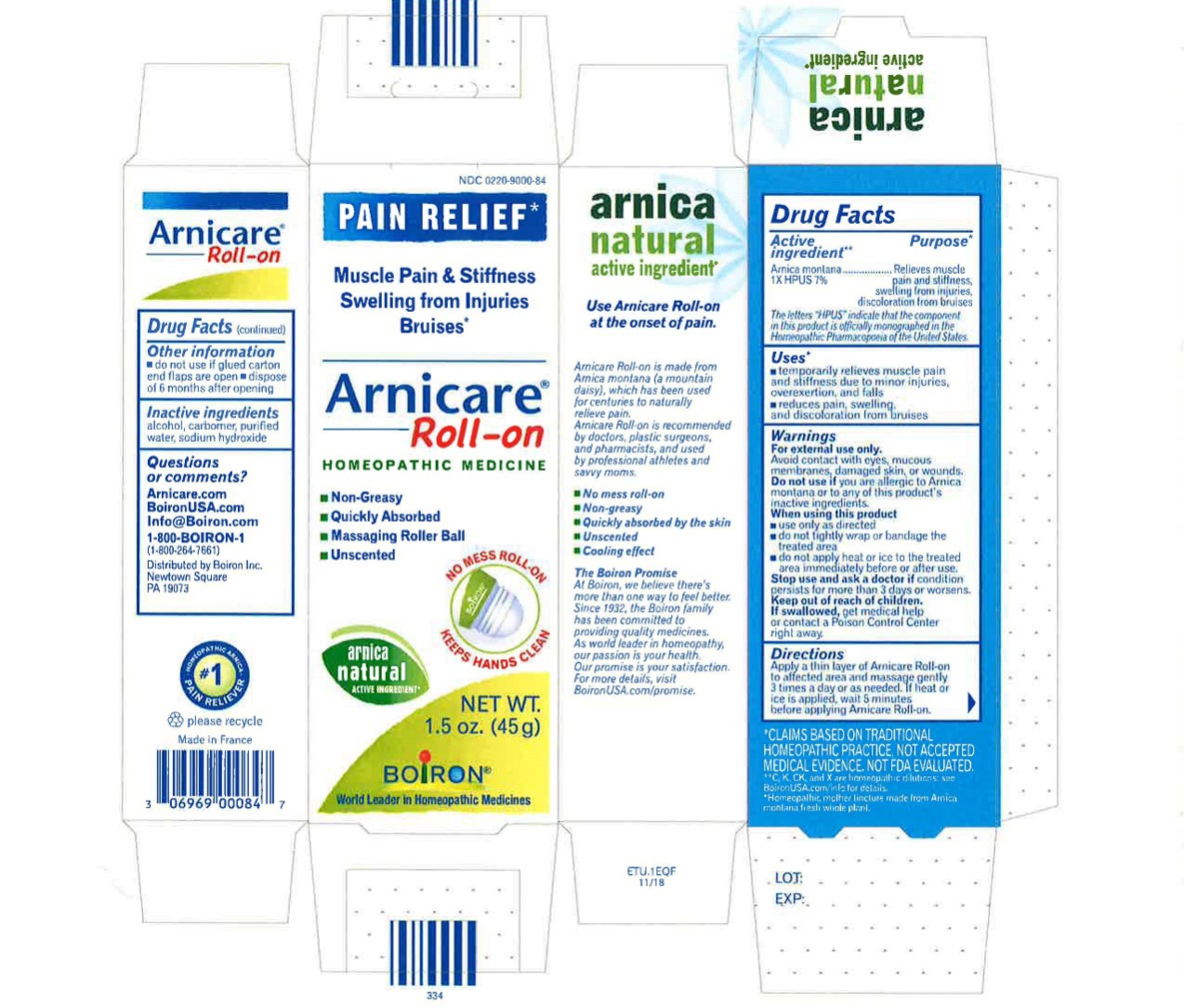
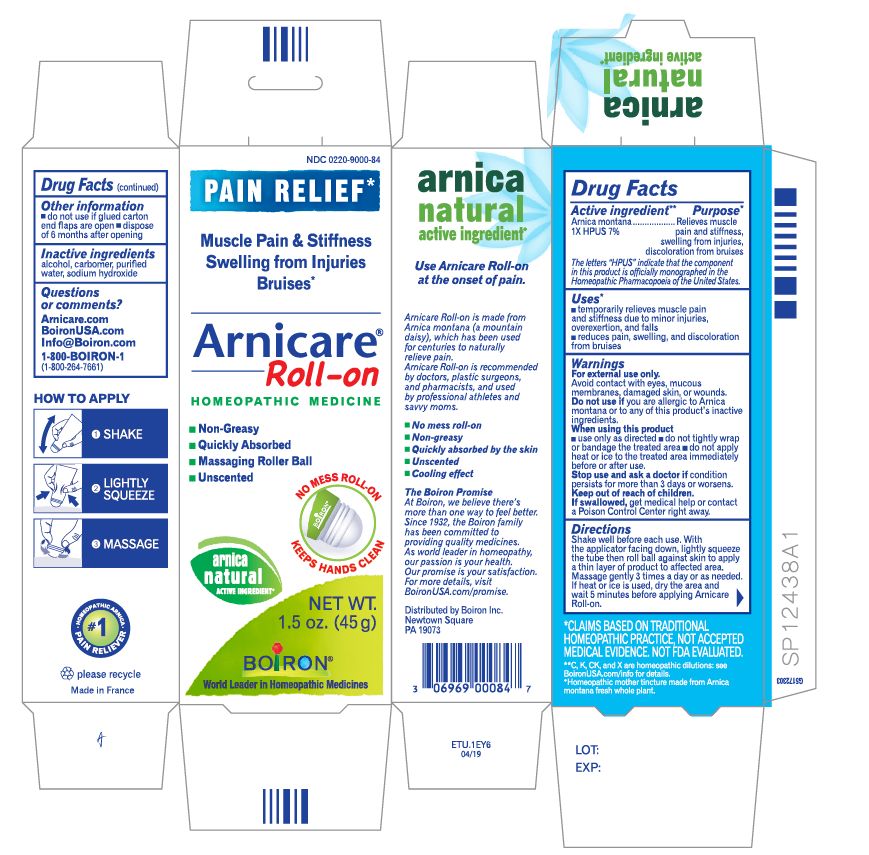
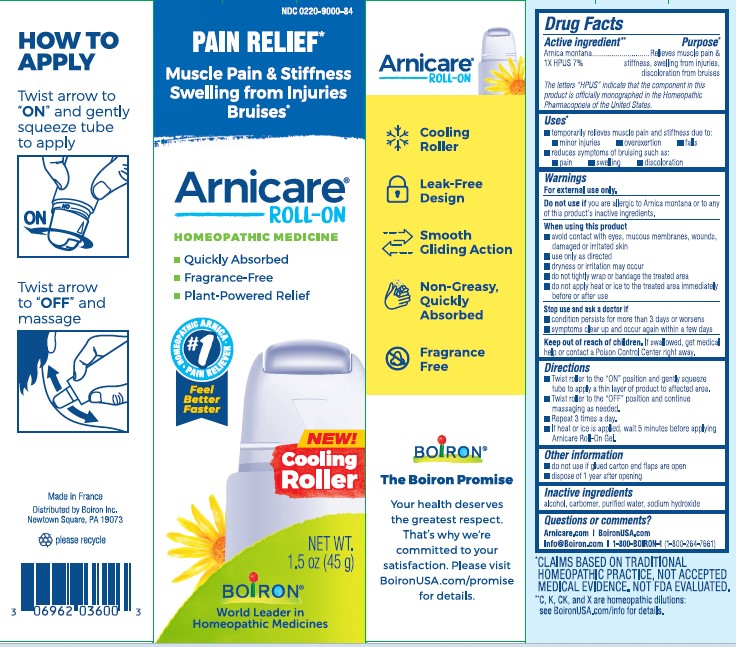
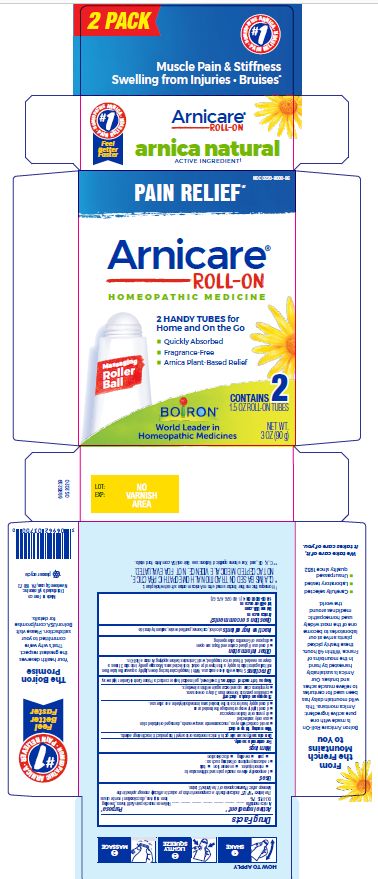
Arnicare Gel Roll-On (2019)- Shake well before each use. With the applicator facing down, lightly squeeze the tube then roll ball against skin to apply a thin layer of the product to affected area. Massage gently into skin 3 times a day or as needed. If heat or ice is applied, wait 5 minutes before applying Arnicare Roll-on.
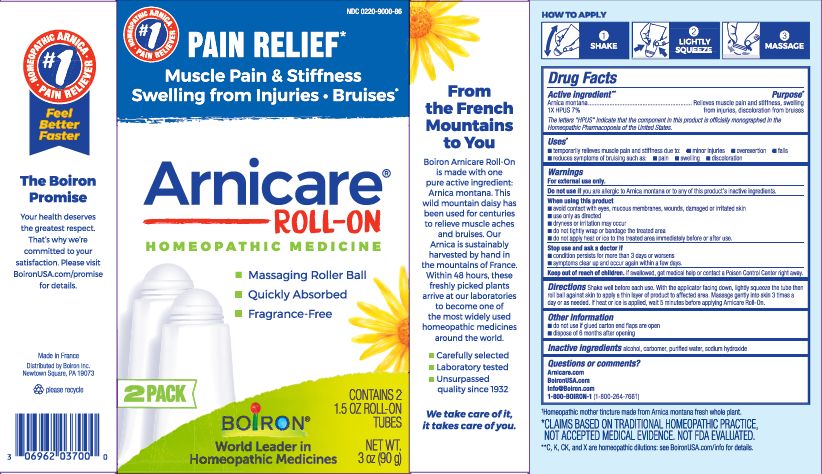
Arnicare Gel Roll-On (2023)- Twist roller to the "ON" position and gently squeeze tube to apply a thin layer of product to affected area. Twist roller to the "OFF" position and continue massaging as needed. Repeat 3 times a day. If heat or ice is applied, wait 5 minutes before applying Arnicare Roll-on Gel.
- INACTIVE INGREDIENT
-
SPL UNCLASSIFIED SECTION
Other information
do not use if glued carton end flaps are open or if the tube seal is broken
do not use if pouch is torn or open
Arnicare Gel 0.18 oz (5g)- dispose 1 day after opening
Arnicare Gel Roll-On (2019)- dispose of 6 months after opening
Arnicare Gel Roll-On (2023)- dispose of 1 year after opening
0.18 oz (5g)
0.5 oz (14g)
1.5 oz (45g)
2.6 oz (75g)
4.2 oz (120g)
3 oz (90g)
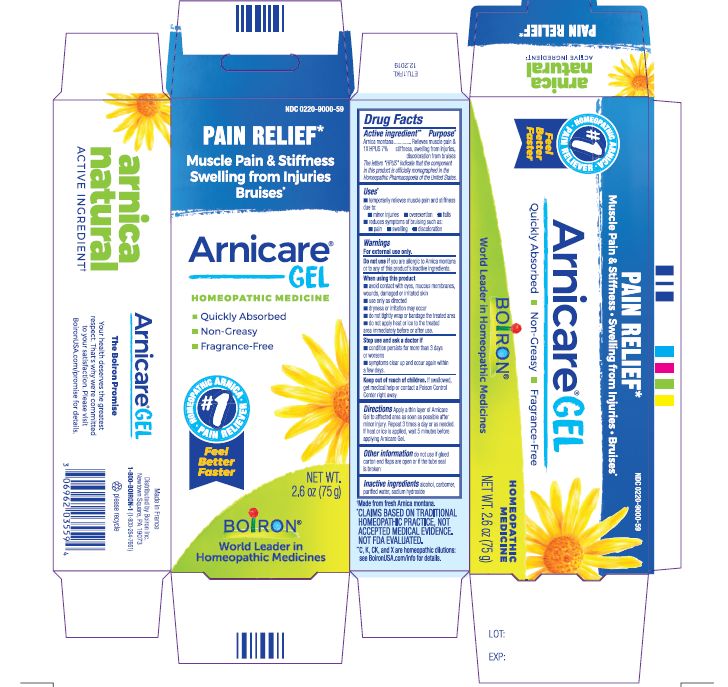
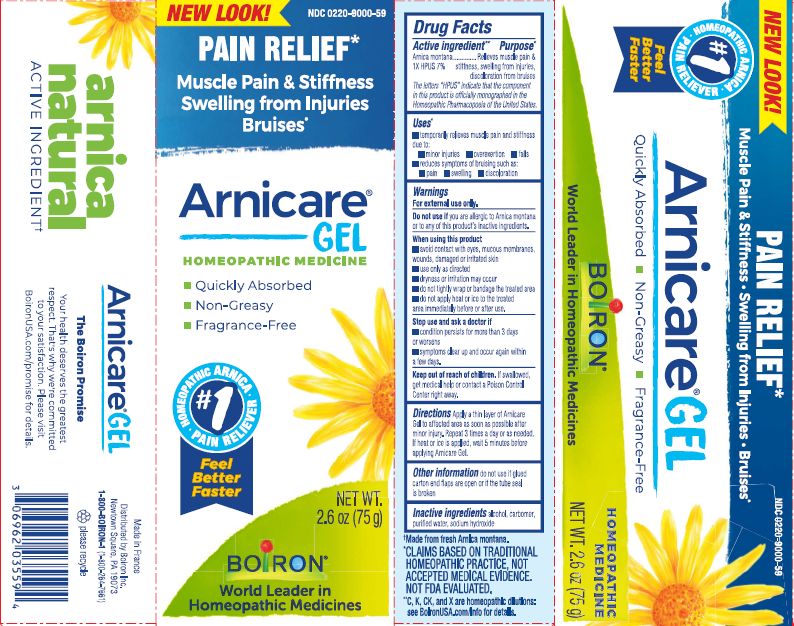
Pain Relief*
Muscle Pain & Stiffness Swelling from Injuries Bruises*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICARE
arnica montana gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9000-66 1 in 1 PACKAGE 01/09/2007 1 120 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0220-9000-59 1 in 1 PACKAGE 01/09/2007 2 75 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:0220-9000-54 1 in 1 PACKAGE 01/09/2007 3 45 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:0220-9000-65 1 in 1 PACKAGE 01/09/2007 4 14 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC:0220-9000-84 1 in 1 PACKAGE 06/01/2018 5 45 g in 1 TUBE; Type 0: Not a Combination Product 6 NDC:0220-9000-86 2 in 1 PACKAGE 06/01/2018 6 45 g in 1 TUBE; Type 0: Not a Combination Product 7 NDC:0220-9000-94 5 g in 1 POUCH; Type 0: Not a Combination Product 06/09/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/09/2007 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9000)