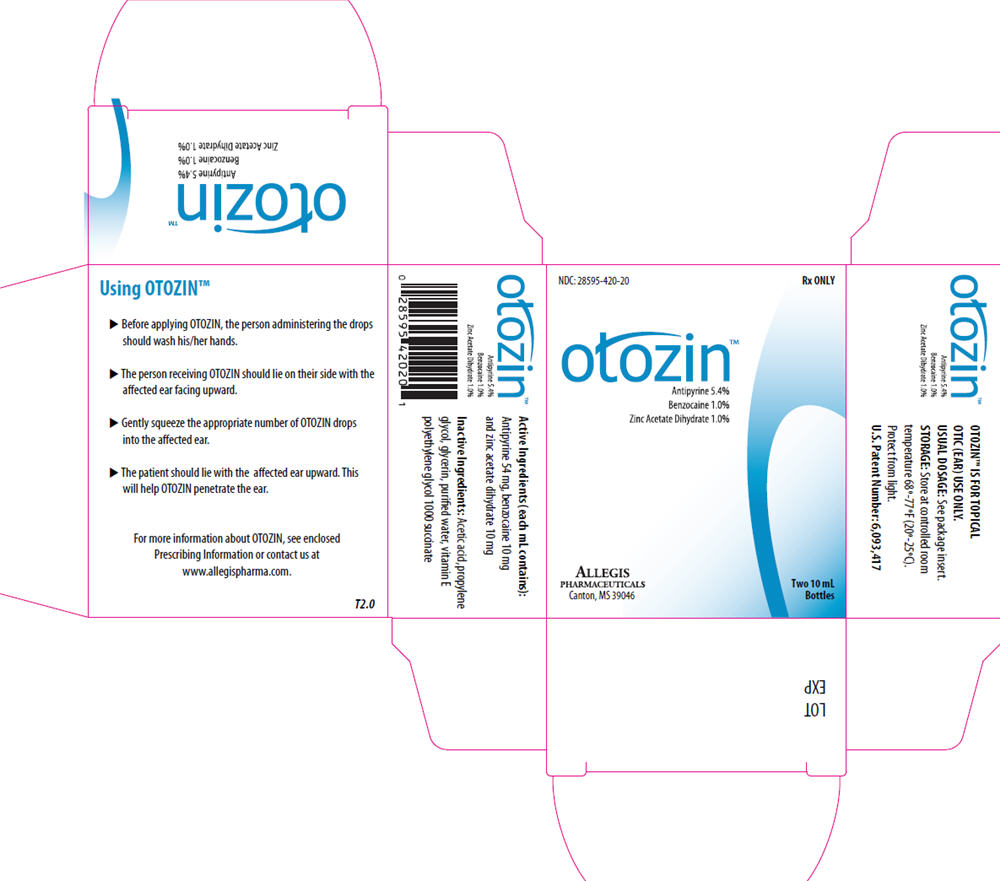
OTOZIN- antipyrine, benzocaine and zinc acetate solution
Allegis Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
otozin™
Antipyrine 5.4%
Bemzpcaome 1.0%
Zinc Acetate Dihydrate 1.0%
DESCRIPTION
Each 1 mL for otic administration contains:
| Antipyrine | 54 mg |
| Benzocaine | 10 mg |
| Zinc Acetate Dihydrate | 10 mg |
Also contains acetic acid, propylene glycol, glycerin, purified water, and vitamin E polyethylene glycol 1000 succinate.
Antipyrine is an antipyretic and analgesic. Antipyrine occurs as a colorless, almost odorless, crystalline powder or tabular crystals having a slightly bitter taste. It is very soluble in water and freely soluble in alcohol. Chemically its structure is C11H12N20.
Benzocaine is a short-acting local anesthetic. Benzocaine occurs as a white, crystalline powder. Chemically its structure is C9H11NO2.
Zinc acetate in the dihydrate form is a salt of zinc and a topical skin protectant generally recognized as safe and effective. Zinc acetate dihydrate occurs as white crystals or granules, is freely soluble in water and in boiling alcohol and is slightly soluble in alcohol. Chemically its structural formula is C4H6O4Zn•2H2O.
INDICATIONS AND USAGE
Acute otitis media of various etiologies
- Prompt relief of pain and reduction of inflammation in the congestive and serous stages.
- Adjuvant therapy during systemic antibiotic administration for resolution of the infection.
Because of the close anatomical relationship of the Eustachian tube to the nasal cavity, otitis media is a frequent problem especially in children in whom the tube is shorter, wider and more horizontal than in adults.
CONTRAINDICATIONS
This product is contraindicated in any person with hypersensitivity to any of the components or substances related to them. This product is contraindicated in the presence of spontaneous perforation of the tympanic membrane or discharge.
PRECAUTIONS
Information for Patients
Avoid contaminating the dropper with material from the ear, fingers or other source.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals or humans have been conducted.
Pregnancy Category C
Animal reproduction studies have not been conducted with this preparation. It is also not known whether this product can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. This product should be given to a pregnant woman only if clearly needed.
DOSAGE AND ADMINISTRATION
Acute otitis media
Warm to body temperature. Using a single bottle dropper, instill the product permitting the solution to run along the wall of the canal until it is filled. Avoid touching the ear with the dropper. Repeat every one to two hours until pain and congestion are relieved.
HOW SUPPLIED
OTOZIN is supplied as a clear liquid delivered in 2 white dropper bottles containing 10 mL each as a single prescription package (NDC 28595-420-20).
| OTOZIN
antipyrine, benzocaine, and zinc acetate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allegis Pharmaceuticals, LLC (792272861) |