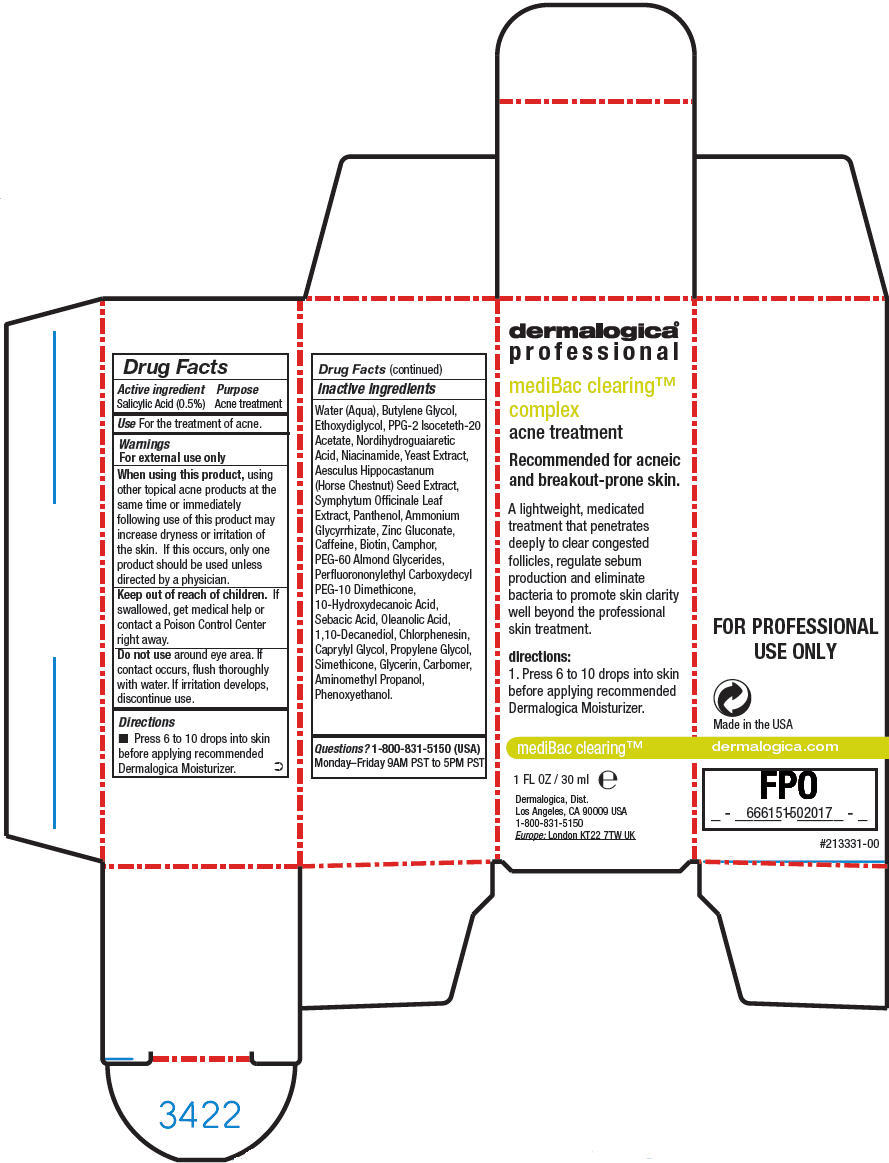
Label: MEDIBAC CLEARING COMPLEX- salicylic acid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 68479-804-02 - Packager: Dermalogica, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 24, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product, using other topical acne products at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one product should be used unless directed by a physician.
- Directions
-
Inactive ingredients
Water (Aqua), Butylene Glycol, Ethoxydiglycol, PPG-2 Isoceteth-20 Acetate, Nordihydroguaiaretic Acid, Niacinamide, Yeast Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Symphytum Officinale Leaf Extract, Panthenol, Ammonium Glycyrrhizate, Zinc Gluconate, Caffeine, Biotin, Camphor, PEG-60 Almond Glycerides, Perfluorononylethyl Carboxydecyl PEG-10 Dimethicone, 10-Hydroxydecanoic Acid, Sebacic Acid, Oleanolic Acid, 1,10-Decanediol, Chlorphenesin, Caprylyl Glycol, Propylene Glycol, Simethicone, Glycerin, Carbomer, Aminomethyl Propanol, Phenoxyethanol.
- Questions?
-
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
dermalogica®
professionalmediBac clearing™
complexacne treatment
Recommended for acneic
and breakout-prone skin.A lightweight, medicated
treatment that penetrates
deeply to clear congested
follicles, regulate sebum
production and eliminate
bacteria to promote skin clarity
well beyond the professional
skin treatment.directions:
1. Press 6 to 10 drops into skin
before applying recommended
Dermalogica Moisturizer.mediBac clearing™
1 FL OZ / 30 ml e
Dermalogica, Dist.
Los Angeles, CA 90009 USA
1-800-831-5150
Europe: London KT22 7TW UK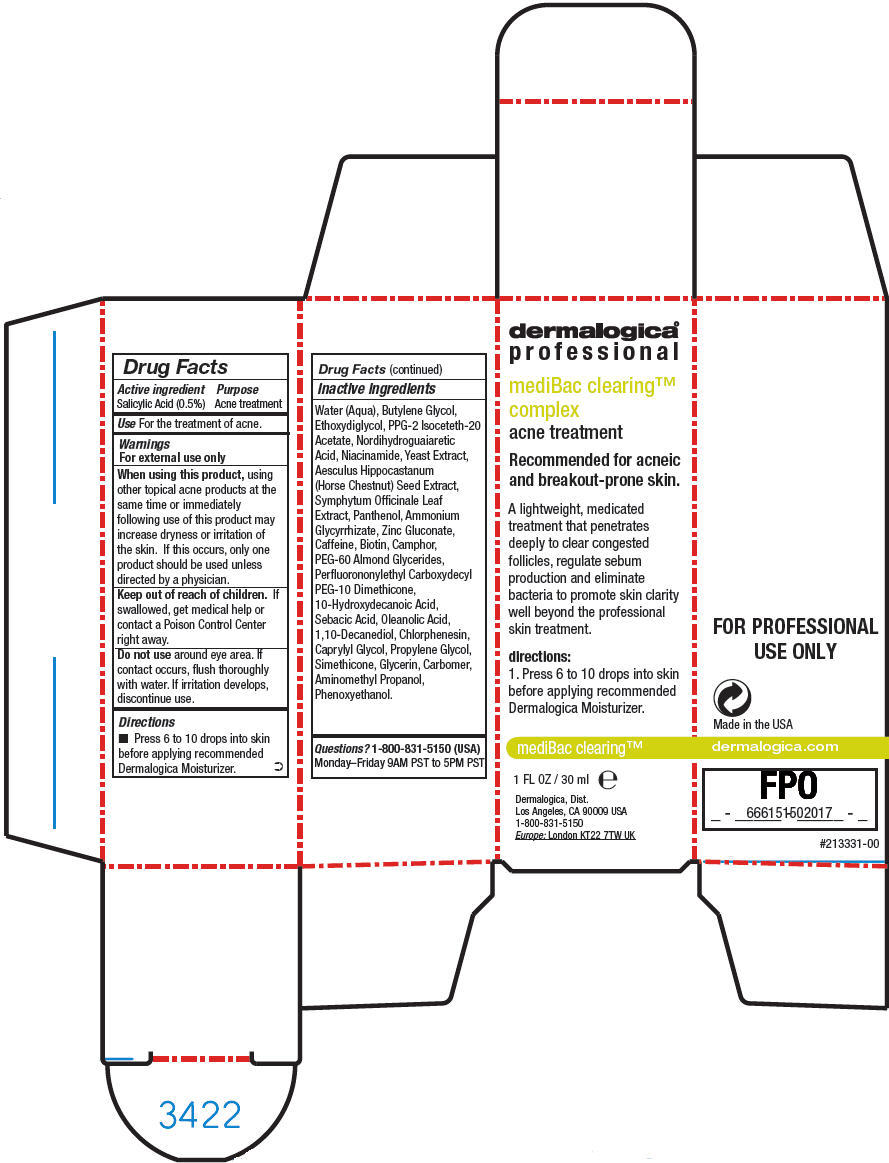
-
INGREDIENTS AND APPEARANCE
MEDIBAC CLEARING COMPLEX
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-804 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) Diethylene Glycol Monoethyl Ether (UNII: A1A1I8X02B) PPG-2 Isoceteth-20 Acetate (UNII: BI6C7YO419) Masoprocol (UNII: 7BO8G1BYQU) Niacinamide (UNII: 25X51I8RD4) Yeast, unspecified (UNII: 3NY3SM6B8U) Horse Chestnut (UNII: 3C18L6RJAZ) Panthenol (UNII: WV9CM0O67Z) Ammonium Glycyrrhizate (UNII: 3VRD35U26C) Zinc Gluconate (UNII: U6WSN5SQ1Z) Caffeine (UNII: 3G6A5W338E) Biotin (UNII: 6SO6U10H04) Comfrey Leaf (UNII: DG4F8T839X) Camphor (Synthetic) (UNII: 5TJD82A1ET) PEG-60 Almond Glycerides (UNII: 4Y0E651N0F) 10-Hydroxydecanoic Acid (UNII: NP03XO416B) Sebacic Acid (UNII: 97AN39ICTC) Oleanolic Acid (UNII: 6SMK8R7TGJ) 1,10-Decanediol (UNII: 5I577UDK52) Chlorphenesin (UNII: I670DAL4SZ) Caprylyl Glycol (UNII: 00YIU5438U) Propylene Glycol (UNII: 6DC9Q167V3) Glycerin (UNII: PDC6A3C0OX) Carbomer Homopolymer Type C (Allyl Pentaerythritol Crosslinked) (UNII: 4Q93RCW27E) Aminomethylpropanol (UNII: LU49E6626Q) Phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-804-02 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 05/09/2007 Labeler - Dermalogica, Inc. (177698560) Establishment Name Address ID/FEI Business Operations PakLab 790530976 MANUFACTURE(68479-804)