NIZATIDINE- nizatidine capsule
Bryant Ranch Prepack
----------
NIZATIDINE CAPSULES
DESCRIPTION
Nizatidine, USP is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine.
The structural formula is as follows:
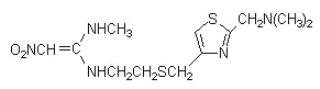
Nizatidine has the empirical formula C12H21N5O2S2 representing a molecular weight of 331.46. It is an off white to buff crystalline solid that is soluble in water. Nizatidine has a bitter taste and mild sulfur-like odor. Each capsule contains for oral administration 150 mg (0.45 mmol) or 300 mg (0.91 mmol) of nizatidine and the following inactive ingredients: croscarmellose sodium, povidone, starch, dimethicone and talc. The capsule shells contain D&C yellow no. 10, Titanium Dioxide, Gelatin, D&C red no. 28, FD&C blue no. 1, FD&C red no. 40 (for 150 mg) and Titanium Dioxide, Gelatin, D&C yellow no.10, FD&C blue no.1, FD&C red no. 40 (for 300 mg). The imprinting ink contains Shellac, Iron Oxide Black, N-Butyl Alcohol, Propylene Glycol, FD&C blue no. 2, FD&C red no. 40, FD&C blue no. 1, D&C yellow no. 10, SDA 3A Alcohol (for 150 mg) and Shellac, Dehydrated Alcohol, Isopropyl Alcohol, Butyl Alcohol, Propylene Glycol, Strong Ammonia Solution, Black Iron Oxide, Potassium Hydroxide (for 300 mg).
CLINICAL PHARMACOLOGY
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells.
Antisecretory Activity
1. Effects on Acid Secretion
Nizatidine significantly inhibited nocturnal gastric acid secretion for up to 12 hours. Nizatidine also significantly inhibited gastric acid secretion stimulated by food, caffeine, betazole, and pentagastrin (Table 1).
| Time After Dose (h) | % Inhibition of Gastric Acid Output by Dose (mg) | |||||
|---|---|---|---|---|---|---|
| 20-50 | 75 | 100 | 150 | 300 | ||
| Nocturnal | Up to 10 | 57 | 73 | 90 | ||
| Betazole | Up to 3 | 93 | 100 | 99 | ||
| Pentagastrin | Up to 6 | 25 | 64 | 67 | ||
| Meal | Up to 4 | 41 | 64 | 98 | 97 | |
| Caffeine | Up to 3 | 73 | 85 | 96 | ||
2. Effects on Other Gastrointestinal Secretions
Pepsin
Oral administration of 75 to 300 mg of nizatidine did not affect pepsin activity in gastric secretions. Total pepsin output was reduced in proportion to the reduced volume of gastric secretions.
3. Other Pharmacologic Actions
- a.
- Hormones: Nizatidine was not shown to affect the serum concentrations of gonadotropins, prolactin, growth hormone, antidiuretic hormone, cortisol, triiodothyronine, thyroxin, testosterone, 5α-dihydrotestosterone, androstenedione, or estradiol.
- b.
- Nizatidine had no demonstrable antiandrogenic action.
4. Pharmacokinetics
The absolute oral bioavailability of nizatidine exceeds 70%. Peak plasma concentrations (700 to 1,800 mcg/L for a 150-mg dose and 1,400 to 3,600 mcg/L for a 300-mg dose) occur from 0.5 to 3 hours following the dose. A concentration of 1,000 mcg/L is equivalent to 3 µmol/L; a dose of 300 mg is equivalent to 905 µmoles. Plasma concentrations 12 hours after administration are less than 10 mcg/L. The elimination half-life is 1 to 2 hours, plasma clearance is 40 to 60 L/h, and the volume of distribution is 0.8 to 1.5 L/kg. Because of the short half-life and rapid clearance of nizatidine, accumulation of the drug would not be expected in individuals with normal renal function who take either 300 mg once daily at bedtime or 150 mg twice daily. Nizatidine exhibits dose proportionality over the recommended dose range.
The oral bioavailability of nizatidine is unaffected by concomitant ingestion of propantheline. Antacids consisting of aluminum and magnesium hydroxides with simethicone decrease the absorption of nizatidine by about 10%. With food, the AUC and Cmax increase by approximately 10%.
In humans, less than 7% of an oral dose is metabolized as N2-monodesmethylnizatidine, an H2-receptor antagonist, which is the principal metabolite excreted in the urine. Other likely metabolites are the N2-oxide (less than 5% of the dose) and the S-oxide (less than 6% of the dose).
More than 90% of an oral dose of nizatidine is excreted in the urine within 12 hours. About 60% of an oral dose is excreted as unchanged drug. Renal clearance is about 500 mL/min, which indicates excretion by active tubular secretion. Less than 6% of an administered dose is eliminated in the feces.
Moderate to severe renal impairment significantly prolongs the half-life and decreases the clearance of nizatidine. In individuals who are functionally anephric, the half-life is 3.5 to 11 hours, and the plasma clearance is 7 to 14 L/h. To avoid accumulation of the drug in individuals with clinically significant renal impairment, the amount and/or frequency of doses of nizatidine should be reduced in proportion to the severity of dysfunction (see DOSAGE AND ADMINISTRATION).
Approximately 35% of nizatidine is bound to plasma protein, mainly to α1-acid glycoprotein. Warfarin, diazepam, acetaminophen, propantheline, phenobarbital, and propranolol did not affect plasma protein binding of nizatidine in vitro.
Clinical Trials
1. Active Duodenal Ulcer
In multicenter, double-blind, placebo-controlled studies in the United States, endoscopically diagnosed duodenal ulcers healed more rapidly following administration of nizatidine, 300 mg h.s. or 150 mg b.i.d., than with placebo (Table 2). Lower doses, such as 100 mg h.s., had slightly lower effectiveness.
| Nizatidine | Placebo | |||||
|---|---|---|---|---|---|---|
| 300 mgh.s. | 150 mgb.i.d. | |||||
| Number Entered | Healed/ Evaluable | Number Entered | Healed/ Evaluable | Number Entered | Healed/ Evaluable |
|
| STUDY 1 | ||||||
| Week 2 | 276 | 93/265 (35%)* | 279 | 55/260 (21%) | ||
| Week 4 | 198/259 (76%)* | 95/243 (39%) | ||||
| STUDY 2 | ||||||
| Week 2 | 108 | 24/103 (23%)* | 106 | 27/101 (27%)* | 101 | 9/93 (10%) |
| Week 4 | 65/97 (67%)* | 66/97 (68%)* | 24/84 (29%) | |||
| STUDY 3 | ||||||
| Week 2 | 92 | 22/90 (24%)† | 98 | 13/92 (14%) | ||
| Week 4 | 52/85 (61%)* | 29/88 (33%) | ||||
| Week 8 | 68/83 (82%)* | 39/79 (49%) | ||||
2. Maintenance of Healed Duodenal Ulcer
Treatment with a reduced dose of nizatidine has been shown to be effective as maintenance therapy following healing of active duodenal ulcers. In multicenter, double-blind, placebo-controlled studies conducted in the United States, 150 mg of nizatidine taken at bedtime resulted in a significantly lower incidence of duodenal ulcer recurrence in patients treated for up to 1 year (Table 3).
| Month | Nizatidine, 150 mg h.s. | Placebo |
|---|---|---|
|
||
| 3 | 13% (28/208)* | 40% (82/204) |
| 6 | 24% (45/188)* | 57% (106/187) |
| 12 | 34% (57/166)* | 64% (112/175) |
3. Gastroesophageal Reflux Disease (GERD)
In 2 multicenter, double-blind, placebo-controlled clinical trials performed in the United States and Canada, nizatidine was more effective than placebo in improving endoscopically diagnosed esophagitis and in healing erosive and ulcerative esophagitis.
In patients with erosive or ulcerative esophagitis, 150 mg b.i.d. of nizatidine given to 88 patients compared with placebo in 98 patients in Study 1 yielded a higher healing rate at 3 weeks (16% vs 7%) and at 6 weeks (32% vs 16%, P<0.05). Of 99 patients on nizatidine and 94 patients on placebo, Study 2 at the same dosage yielded similar results at 6 weeks (21% vs 11%, P<0.05) and at 12 weeks (29% vs 13%, P<0.01).
In addition, relief of associated heartburn was greater in patients treated with nizatidine. Patients treated with nizatidine consumed fewer antacids than did patients treated with placebo.
4. Active Benign Gastric Ulcer
In a multicenter, double-blind, placebo-controlled study conducted in the United States and Canada, endoscopically diagnosed benign gastric ulcers healed significantly more rapidly following administration of nizatidine than of placebo (Table 4).
| Week | Treatment | Healing Rate | vs. Placebo p-value* |
|---|---|---|---|
|
|||
| 4 | Niz 300 mg h.s. | 52/153 (34%) | 0.342 |
| Niz 150 mg b.i.d. | 65/151 (43%) | 0.022 | |
| Placebo | 48/151 (32%) | ||
| 8 | Niz 300 mg h.s. | 99/153 (65%) | 0.011 |
| Niz 150 mg b.i.d. | 105/151 (70%) | <0.001 | |
| Placebo | 78/151 (52%) | ||
In a multicenter, double-blind, comparator-controlled study in Europe, healing rates for patients receiving nizatidine (300 mg h.s. or 150 mg b.i.d.) were equivalent to rates for patients receiving a comparator drug, and statistically superior to historical placebo control rates.
INDICATIONS AND USAGE
Nizatidine capsules are indicated for up to 8 weeks for the treatment of active duodenal ulcer. In most patients, the ulcer will heal within 4 weeks.
Nizatidine capsules are indicated for maintenance therapy for duodenal ulcer patients at a reduced dosage of 150 mg h.s. after healing of an active duodenal ulcer. The consequences of continuous therapy with nizatidine for longer than 1 year are not known.
Nizatidine capsules are indicated for up to 12 weeks for the treatment of endoscopically diagnosed esophagitis, including erosive and ulcerative esophagitis, and associated heartburn due to GERD.
Nizatidine capsules are indicated for up to 8 weeks for the treatment of active benign gastric ulcer. Before initiating therapy, care should be taken to exclude the possibility of malignant gastric ulceration.
CONTRAINDICATION
Nizatidine capsules are contraindicated in patients with known hypersensitivity to the drug. Because cross sensitivity in this class of compounds has been observed, H2-receptor antagonists, including nizatidine, should not be administered to patients with a history of hypersensitivity to other H2-receptor antagonists.
PRECAUTIONS
General
- Symptomatic response to nizatidine therapy does not preclude the presence of gastric malignancy.
- Because nizatidine is excreted primarily by the kidney, dosage should be reduced in patients with moderate to severe renal insufficiency (see DOSAGE AND ADMINISTRATION).
- Pharmacokinetic studies in patients with hepatorenal syndrome have not been done. Part of the dose of nizatidine is metabolized in the liver. In patients with normal renal function and uncomplicated hepatic dysfunction, the disposition of nizatidine is similar to that in normal subjects.
Laboratory Tests
False-positive tests for urobilinogen with Multistix® may occur during therapy with nizatidine.
Drug Interactions
No interactions have been observed between nizatidine and theophylline, chlordiazepoxide, lorazepam, lidocaine, phenytoin, and warfarin. Nizatidine does not inhibit the cytochrome P-450-linked drug-metabolizing enzyme system; therefore, drug interactions mediated by inhibition of hepatic metabolism are not expected to occur. In patients given very high doses (3,900 mg) of aspirin daily, increases in serum salicylate levels were seen when nizatidine, 150 mg b.i.d., was administered concurrently.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year oral carcinogenicity study in rats with doses as high as 500 mg/kg/day (about 80 times the recommended daily therapeutic dose) showed no evidence of a carcinogenic effect. There was a dose-related increase in the density of enterochromaffin-like (ECL) cells in the gastric oxyntic mucosa. In a 2-year study in mice, there was no evidence of a carcinogenic effect in male mice; although hyperplastic nodules of the liver were increased in the high-dose males as compared with placebo. Female mice given the high dose of nizatidine (2,000 mg/kg/day, about 330 times the human dose) showed marginally statistically significant increases in hepatic carcinoma and hepatic nodular hyperplasia with no numerical increase seen in any of the other dose groups. The rate of hepatic carcinoma in the high-dose animals was within the historical control limits seen for the strain of mice used. The female mice were given a dose larger than the maximum tolerated dose, as indicated by excessive (30%) weight decrement as compared with concurrent controls and evidence of mild liver injury (transaminase elevations). The occurrence of a marginal finding at high dose only in animals given an excessive and somewhat hepatotoxic dose, with no evidence of a carcinogenic effect in rats, male mice, and female mice (given up to 360 mg/kg/day, about 60 times the human dose), and a negative mutagenicity battery are not considered evidence of a carcinogenic potential for nizatidine.
Nizatidine was not mutagenic in a battery of tests performed to evaluate its potential genetic toxicity, including bacterial mutation tests, unscheduled DNA synthesis, sister chromatid exchange, mouse lymphoma assay, chromosome aberration tests, and a micronucleus test.
In a 2-generation, perinatal and postnatal fertility study in rats, doses of nizatidine up to 650 mg/kg/day produced no adverse effects on the reproductive performance of parental animals or their progeny.
Pregnancy
Teratogenic Effects
Pregnancy category B
Oral reproduction studies in pregnant rats at doses up to 1500 mg/kg/day (9000 mg/m2/day, 40.5 times the recommended human dose based on body surface area) and in pregnant rabbits at doses up to 275 mg/kg/day (3245 mg/m2/day, 14.6 times the recommended human dose based on body surface area) have revealed no evidence of impaired fertility or harm to the fetus due to nizatidine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Studies conducted in lactating women have shown that 0.1% of the administered oral dose of nizatidine is secreted in human milk in proportion to plasma concentrations. Because of the growth depression in pups reared by lactating rats treated with nizatidine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Of the 955 patients in clinical studies who were treated with nizatidine, 337 (35.3%) were 65 and older. No overall differences in safety or effectiveness were observed between these and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (seeDOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Worldwide, controlled clinical trials of nizatidine included over 6,000 patients given nizatidine in studies of varying durations. Placebo-controlled trials in the United States and Canada included over 2,600 patients given nizatidine and over 1,700 given placebo. Among the adverse events in these placebo-controlled trials, anemia (0.2% vs 0%) and urticaria (0.5% vs 0.1%) were significantly more common in the nizatidine group.
Incidence in Placebo-Controlled Clinical Trials in the United States and Canada
Table 5 lists adverse events that occurred at a frequency of 1% or more among nizatidine-treated patients who participated in placebo-controlled trials. The cited figures provide some basis for estimating the relative contribution of drug and nondrug factors to the side-effect incidence rate in the population studied.
| Percentage of Patients Reporting Event | ||
|---|---|---|
| Body System/Adverse Event* | Nizatidine (N=2,694) | Placebo (N=1,729) |
|
||
| Body as a Whole | ||
| Headache | 16.6 | 15.6 |
| Abdominal pain | 7.5 | 12.5 |
| Pain | 4.2 | 3.8 |
| Asthenia | 3.1 | 2.9 |
| Back pain | 2.4 | 2.6 |
| Chest pain | 2.3 | 2.1 |
| Infection | 1.7 | 1.1 |
| Fever | 1.6 | 2.3 |
| Surgical procedure | 1.4 | 1.5 |
| Injury, accident | 1.2 | 0.9 |
| Digestive | ||
| Diarrhea | 7.2 | 6.9 |
| Nausea | 5.4 | 7.4 |
| Flatulence | 4.9 | 5.4 |
| Vomiting | 3.6 | 5.6 |
| Dyspepsia | 3.6 | 4.4 |
| Constipation | 2.5 | 3.8 |
| Dry mouth | 1.4 | 1.3 |
| Nausea and vomiting | 1.2 | 1.9 |
| Anorexia | 1.2 | 1.6 |
| Gastrointestinal disorder | 1.1 | 1.2 |
| Tooth disorder | 1.0 | 0.8 |
| Musculoskeletal | ||
| Myalgia | 1.7 | 1.5 |
| Nervous | ||
| Dizziness | 4.6 | 3.8 |
| Insomnia | 2.7 | 3.4 |
| Abnormal dreams | 1.9 | 1.9 |
| Somnolence | 1.9 | 1.6 |
| Anxiety | 1.6 | 1.4 |
| Nervousness | 1.1 | 0.8 |
| Respiratory | ||
| Rhinitis | 9.8 | 9.6 |
| Pharyngitis | 3.3 | 3.1 |
| Sinusitis | 2.4 | 2.1 |
| Cough, increased | 2.0 | 2.0 |
| Skin and Appendages | ||
| Rash | 1.9 | 2.1 |
| Pruritus | 1.7 | 1.3 |
| Special Senses | ||
| Amblyopia | 1.0 | 0.9 |
A variety of less common events were also reported; it was not possible to determine whether these were caused by nizatidine.
Hepatic
Hepatocellular injury, evidenced by elevated liver enzyme tests (SGOT [AST], SGPT [ALT], or alkaline phosphatase), occurred in some patients and was possibly or probably related to nizatidine. In some cases, there was marked elevation of SGOT, SGPT enzymes (greater than 500 IU/L) and, in a single instance, SGPT was greater than 2,000 IU/L. The overall rate of occurrences of elevated liver enzymes and elevations to 3 times the upper limit of normal, however, did not significantly differ from the rate of liver enzyme abnormalities in placebo-treated patients. All abnormalities were reversible after discontinuation of nizatidine. Since market introduction, hepatitis and jaundice have been reported. Rare cases of cholestatic or mixed hepatocellular and cholestatic injury with jaundice have been reported with reversal of the abnormalities after discontinuation of nizatidine.
Cardiovascular
In clinical pharmacology studies, short episodes of asymptomatic ventricular tachycardia occurred in 2 individuals administered nizatidine and in 3 untreated subjects.
Endocrine
Clinical pharmacology studies and controlled clinical trials showed no evidence of antiandrogenic activity due to nizatidine. Impotence and decreased libido were reported with similar frequency by patients who received nizatidine and by those given placebo. Rare reports of gynecomastia occurred.
Hematologic
Anemia was reported significantly more frequently in nizatidine- than in placebo-treated patients. Fatal thrombocytopenia was reported in a patient who was treated with nizatidine and another H2-receptor antagonist. On previous occasions, this patient had experienced thrombocytopenia while taking other drugs. Rare cases of thrombocytopenic purpura have been reported.
Integumental
Sweating and urticaria were reported significantly more frequently in nizatidine than in placebo-treated patients. Rash and exfoliative dermatitis were also reported. Vasculitis has been reported rarely.
Hypersensitivity
As with other H2-receptor antagonists, rare cases of anaphylaxis following administration of nizatidine have been reported. Rare episodes of hypersensitivity reactions (eg, bronchospasm, laryngeal edema, rash, and eosinophilia) have been reported.
OVERDOSAGE
Overdoses of nizatidine have been reported rarely. The following is provided to serve as a guide should such an overdose be encountered.
Signs and Symptoms
There is little clinical experience with overdosage of nizatidine in humans. Test animals that received large doses of nizatidine have exhibited cholinergic-type effects, including lacrimation, salivation, emesis, miosis, and diarrhea. Single oral doses of 800 mg/kg in dogs and of 1,200 mg/kg in monkeys were not lethal. Intravenous median lethal doses in the rat and mouse were 301 mg/kg and 232 mg/kg respectively.
Treatment
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
If overdosage occurs, use of activated charcoal, emesis, or lavage should be considered along with clinical monitoring and supportive therapy. The ability of hemodialysis to remove nizatidine from the body has not been conclusively demonstrated; however, due to its large volume of distribution, nizatidine is not expected to be efficiently removed from the body by this method.
DOSAGE AND ADMINISTRATION
Active Duodenal Ulcer
The recommended oral dosage for adults is 300 mg once daily at bedtime. An alternative dosage regimen is 150 mg twice daily.
Maintenance of Healed Duodenal Ulcer
The recommended oral dosage for adults is 150 mg once daily at bedtime.
Gastroesophageal Reflux Disease
The recommended oral dosage in adults for the treatment of erosions, ulcerations, and associated heartburn is 150 mg twice daily.
Active Benign Gastric Ulcer
The recommended oral dosage is 300 mg given either as 150 mg twice daily or 300 mg once daily at bedtime. Prior to treatment, care should be taken to exclude the possibility of malignant gastric ulceration.
Dosage Adjustment for Patients With Moderate to Severe Renal Insufficiency
The dose for patients with renal dysfunction should be reduced as follows:
| Active Duodenal Ulcer, GERD, and Benign Gastric Ulcer | |
| Ccr | Dose |
| 20-50 mL/min | 150 mg daily |
| <20 mL/min | 150 mg every other day |
| Maintenance Therapy | |
| Ccr | Dose |
| 20-50 mL/min | 150 mg every other day |
| <20 mL/min | 150 mg every 3 days |
Some elderly patients may have creatinine clearances of less than 50 mL/min, and, based on pharmacokinetic data in patients with renal impairment, the dose for such patients should be reduced accordingly. The clinical effects of this dosage reduction in patients with renal failure have not been evaluated.
| NIZATIDINE
nizatidine capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-2769) , RELABEL(63629-2769) | |
