ULTRACARE ORAL ANESTHETIC PINA COLADA- benzocaine gel
Ultradent Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ULTRACARE®
Oral Anesthetic Gel
Uses
for the temporary relief of occasional minor irritation and pain, associated with:
- minor dental procedures
- sore mouth and throat
- minor injury of the gums
- canker sores
- minor irritation of the mouth or gums caused by dentures or orthodontic appliances.
Warning
- For external use only.
Allergy alert
Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Stop use and ask a doctor
- if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea or vomiting
- if sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persists or worsens.
Directions
| Adults and children 2 years of age and older | Apply (a thin film) to affected area. Allow to remain in place at least 1 minute and then spit out. Use up to 4 times daily as directed by a dentist or doctor. |
| Children under 12 years of age | Should be supervised in the use of this product. |
| Children under 2 years of age | Consult a dentist or a doctor. |
Inactive Ingredients
artificial flavor, aspartame, ethyl alcohol, FD&C yellow #5, glycerin, polyethylene glycol, sodium saccharin, titanium dioxide.
ULTRACARE®
Oral Anesthetic Gel
Description:
Ultracare is a 20% benzocaine w/v (oral anesthetic gel) preparation in a water-soluble glycol base. It is available in 30ml bottles, 1.2ml single dose syringes, and in 30ml IndiSpense® container syringes. Special flavoring and sweetening agents are incorporated to render flavors of superior taste (with no aftertaste). Ultracare is designed for rapid onset (10-30 seconds). Anesthesia usually lasts 8-10 minutes. Benzocaine has been shown to be a safe, effective anesthetic with little or no systemic absorption. The incidence of allergic reaction is rare. Ultracare is NOT for injection.
Uses:
For the temporary relief of occasional minor irritation and pain, associated with:
- minor dental procedures
- sore mouth and throat
- minor injury of the gums
- canker sores
- minor irritation of the mouth or gums caused by dentures or orthodontic appliances.
Preliminary Steps:
| 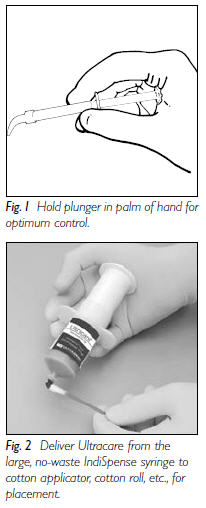 |
Procedure:
- 1.
- Vacuum excess saliva from mouth.
- 2.
- Deliver a controlled amount of Ultracare to desired area.
Precautions:
- 1.
- Do not use Ultracare on patients with history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
- 2.
- Ultracare is NOT for injection.
- 3.
- The 1.2ml syringe size is designed for one-per-patient usage. Dispose syringe and tip following use, or use Ultradent® Syringe Covers sealed with the Impulse Sealer to prevent cross-contamination.
- 4.
- Phenylketonurics: Contains Phenylalanine—3mg per gram.
| Keep out of reach of children.
Store at room temperature. For immediate reorder and/or complete descriptions of Ultradent's product line, refer to Ultradent's catalog or call Toll Free: 1-800-552-5512. Outside U.S. call (801) 572-4200. |  |
| All Ultradent syringes have an expiration date stamped on the side of the syringe consisting of one letter and three numbers. The letter is a lot number used for manufacturing purposes and the three numbers are the expiration date. The first two numbers are the month, and the third number is the last number of the year. | |
All Ultradent syringes have an expiration date stamped on the side of the syringe consisting of one letter and three numbers. The letter is a lot number used for manufacturing purposes and the three numbers are the expiration date. The first two numbers are the month, and the third number is the last number of the year.
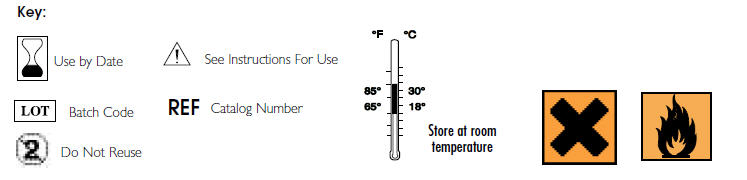
© Copyright 2000 Ultradent Products, Inc. All Rights Reserved.
Ultradent Products, Inc.
505 West 10200 South,
South Jordan, Utah 84095, USA
#10057.7
032107
| ULTRACARE ORAL ANESTHETIC PINA COLADA
benzocaine gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Ultradent Products, Inc. (013369913) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultradent Products, Inc. | 013369913 | MANUFACTURE(51206-204) , ANALYSIS(51206-204) , LABEL(51206-204) , PACK(51206-204) | |
