ANTI-DIARRHEAL LOPERAMIDE HCL- loperamide hydrochloride capsule, liquid filled
Strategic Sourcing Services LLC
----------
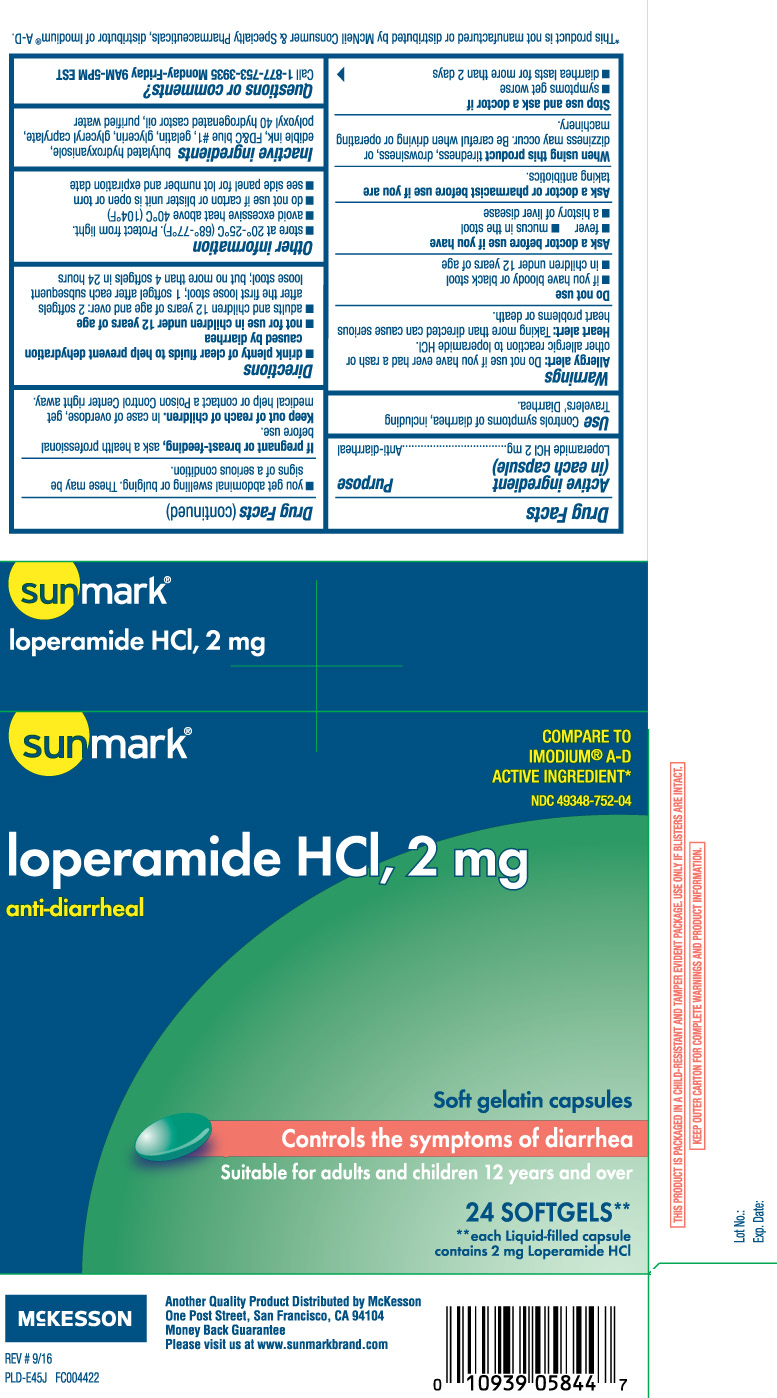
DRUG FACTS
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.
When using this product
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- not for use in children under 12 years of age
- adults and children 12 years and over: 2 softgels after the first loose stool; 1 softgel after each subsequent loose stool; but no more than 4 softgels in 24 hours
Other information
- store at 20o-25oC (68o-77o F) Protect from light.
- avoid excessive heat above 40°C (104°F).
- do not use if carton or blister unit is open or torn
- see side panel for lot number and expiration date
Inactive Ingredients
butylated hydroxyanisole, edible ink, FDandC Blue #1, gelatin, glycerin, glyceryl caprylate, polyoxyl 40 hydrogenated castor oil and purified water.
Principal Display Panel
COMPARE TO IMODIUM® A-D ACTIVE INGREDIENT*
loperamide HCI, 2 mg
Anti-Diarrheal
Soft Gelatin Capsules
Controls the symptoms of diarrhea
Suitable for adults and children 12 years and over
SOFTGELS**
**each Liquid-capsule contains 2 mg Loperamide HCl
THIS PRODUCT IS PACKAGED IN A CHILD RESISTANT AND TAMPER EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
**This product is not manufactured or distributed by McNeil Consumer & Specialty Pharmaceuticals, distributor of Imodium® A-D.
Another Quality Product Distributed by McKesson
One Post Street, San Francisco, CA 94104
Please visit us at www.sunmarkbrand.com
| ANTI-DIARRHEAL
LOPERAMIDE HCL
loperamide hydrochloride capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |