BABIES R US GAS RELIEF INFANTS- simethicone emulsion
Toys"R"Us, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
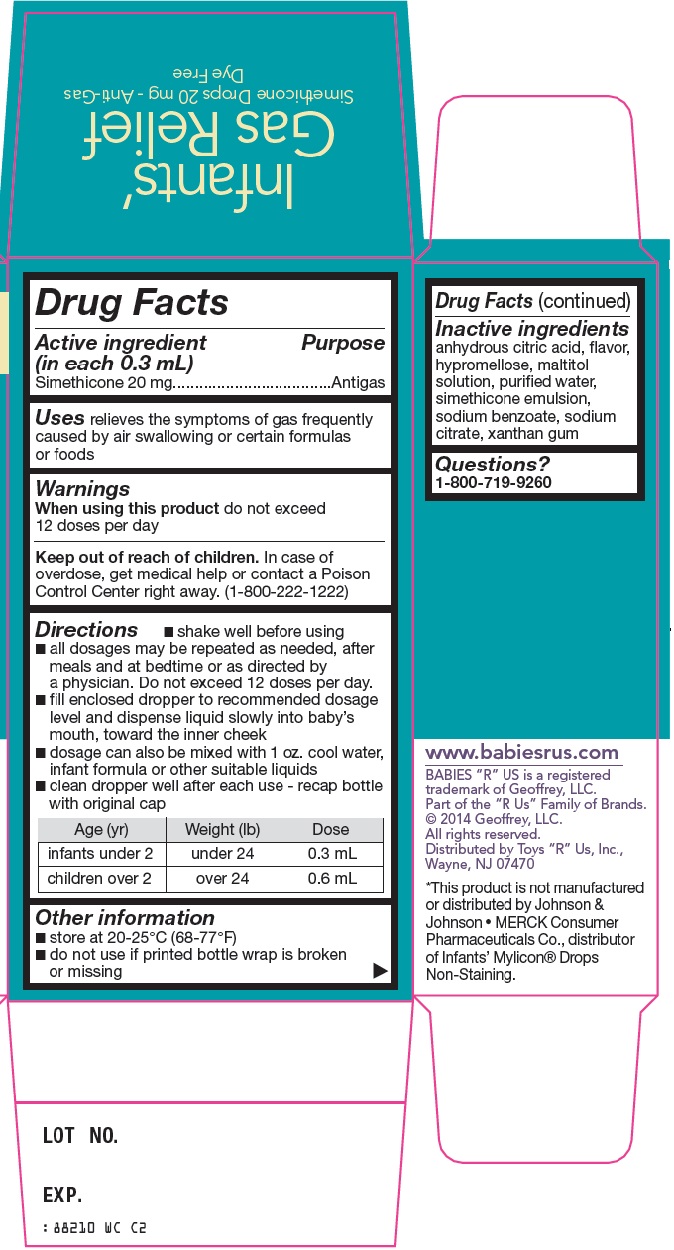
Toys "R" Us, Inc. Infants' Gas Relief Drug Facts
Directions
- •
- shake well before using
- •
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
- •
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby’s mouth, toward the inner cheek
- •
- dosage can also be mixed with 1 oz. cool water, infant formula or other suitable liquids
- •
- clean dropper well after each use – recap bottle with original cap
|
Age (yr) |
Weight (lb) |
Dose |
|
infants under 2 |
under 24 |
0.3 mL |
|
children over 2 |
over 24 |
0.6 mL |
Other information
- •
- store at 20-25°C (68-77°F)
- •
- do not use if printed bottle wrap is broken or missing
| BABIES R US GAS RELIEF
INFANTS
simethicone emulsion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Toys"R"Us, Inc. (006985808) |
Revised: 11/2017
Document Id: 52bd4c2d-df1c-4c50-950b-a5a728b05b36
Set id: cb8770ca-73bb-438a-88c5-65e0c326bfa2
Version: 3
Effective Time: 20171125
Toys"R"Us, Inc.