SCALPICIN 2 IN 1- salicylic acid liquid
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
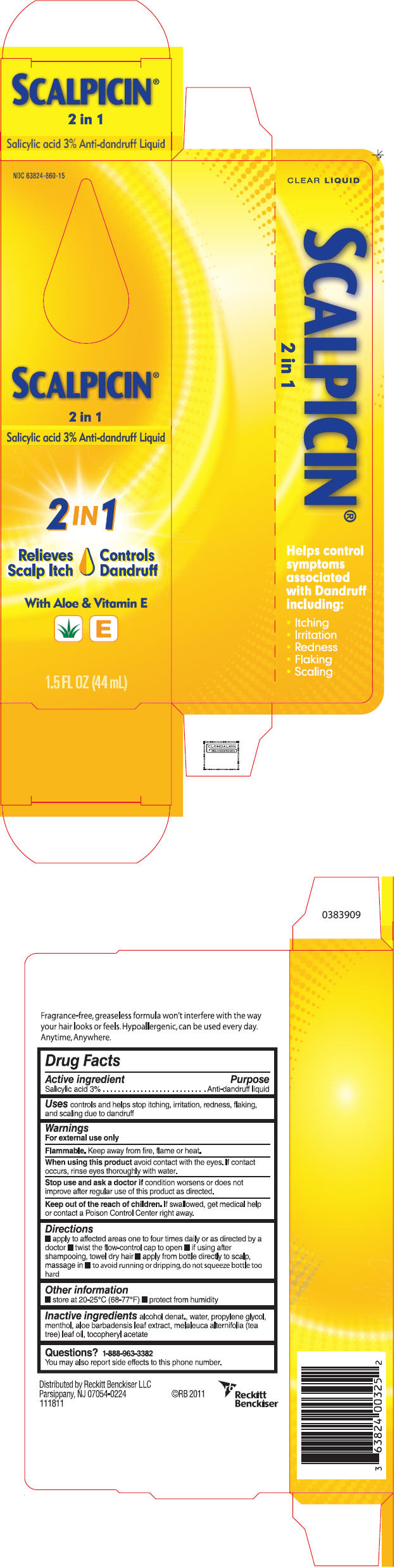
Scalpicin® 2 in 1
Warnings
For external use only
Flammable. Keep away from fire, flame or heat.
When using this product avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- apply to affected areas one to four times daily or as directed by a doctor
- twist the flow-control cap to open
- if using after shampooing, towel dry hair
- apply from bottle directly to scalp, massage in
- to avoid running or dripping, do not squeeze bottle too hard
| SCALPICIN 2 IN 1
salicylic acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |
Revised: 11/2019
Document Id: 5699a418-478a-4272-9bc4-5d0fb2bc345d
Set id: cae9f0a6-a0d2-4170-8d18-2d234349b015
Version: 2
Effective Time: 20191121
RB Health (US) LLC