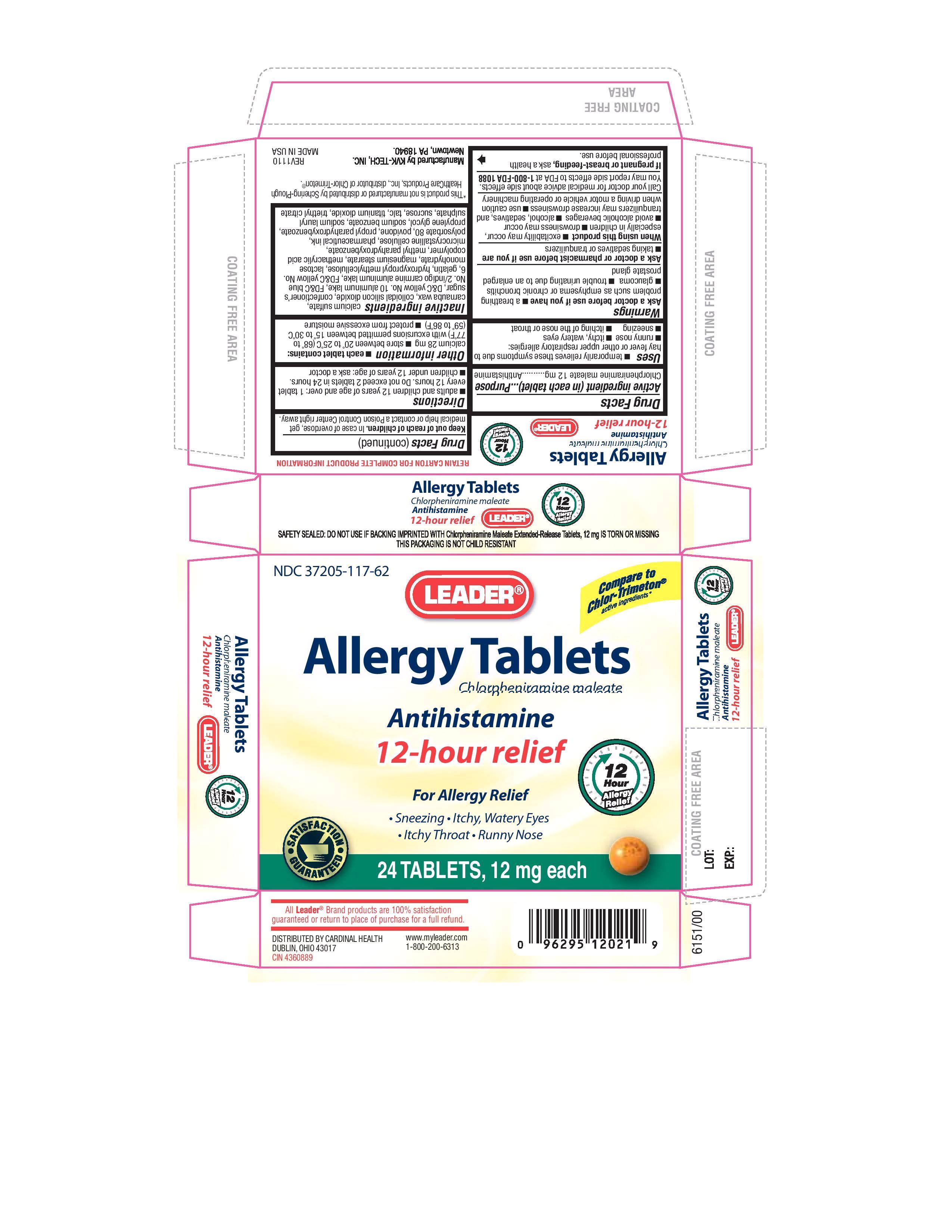
Label: CHLORPHENIRAMINE MALEATE tablet, film coated, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 37205-117-62 - Packager: Cardinal Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 30, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Chlorpheniramine maleate 12 mg
- USES
-
WARNINGS
Ask a doctor before use if you have
a breathing problem such as emphysema or chronic bronchitis
glaucoma
trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
excitability may occur, especially in children
drowsiness may occur
avoid alcoholic beverages
alchohol, sedatives and tranquilizers may increase drowsiness
use caution when driving a motor vehicle or operating machinery
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088
If pregnant or breast-feeding, ask a health professional before use
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
-
calcium sulfate, carnauba wax, colloidal silicon dioxide, confectioner’s sugar, D&C yellow No. 10 aluminum lake, FD&C blue No. 2/indigo carmine aluminum lake, FD&C yellow No. 6, gelatin, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, methacrylic acid copolymer, methyl parahydroxybenzoate, microcrystalline cellulose, pharmaceutical ink, polysorbate 80, povidone, propyl parahydroxybenzoate, propylene glycol, sodium benzoate, sodium lauryl sulphate, sucrose, talc, titanium dioxide, triethyl citrate
-
- PURPOSE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHLORPHENIRAMINE MALEATE
chlorpheniramine maleate tablet, film coated, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37205-117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 12 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE (UNII: WAT0DDB505) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCROSE (UNII: C151H8M554) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GELATIN (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) METHYLPARABEN (UNII: A2I8C7HI9T) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color BROWN Score no score Shape ROUND Size 7mm Flavor Imprint Code Allergy;12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37205-117-62 24 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040829 12/20/2010 Labeler - Cardinal Health, Inc. (097537435) Registrant - KVK-Tech, Inc. (173360061) Establishment Name Address ID/FEI Business Operations KVK-Tech, Inc. 173360061 MANUFACTURE(37205-117)