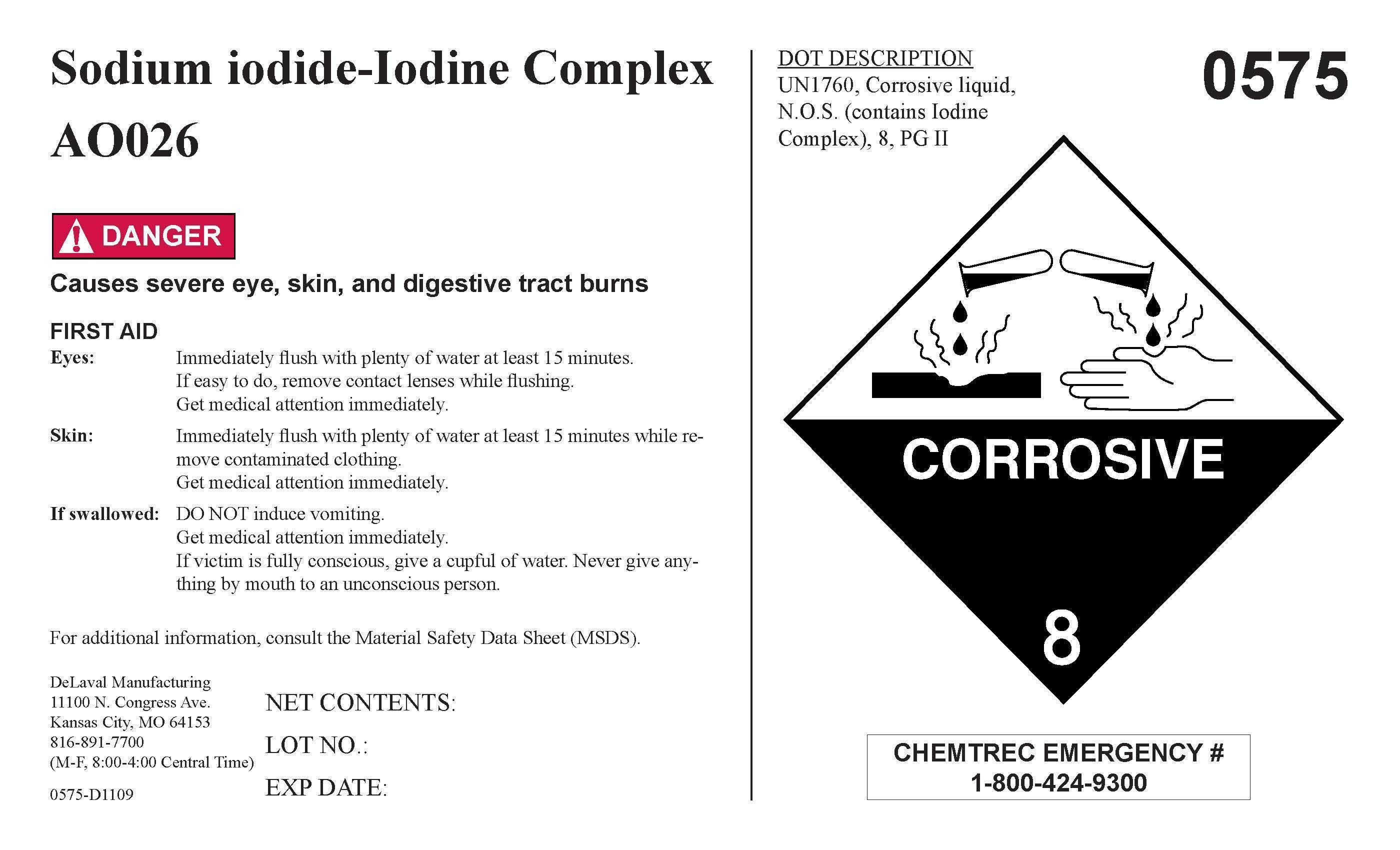
SODIUM IODIDE-IODINE COMPLEX- iodine concentrate
WestAgro, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sodium iodide-Iodine Complex A0026
FIRST AID
Eyes: Immediately flush with plenty of water at least 15
minutes. If easy to do, remove contact lenses while flushing. Get medical attention immediately.
Skin: Immediately flush with plenty of water at least 15 minutes while removing contaminated clothing. Get medical attention immediately.
If swallowed: DO NOT induce vomiting. Get medical attention immediately. If victim is fully conscious, give a cupful of water. Never give anything my mouth to an unconscious person.
For additional information, consult the Material Safety Data Sheet (MSDS).
DeLaval Manufacturing
11100 N. Congress Ave.
Kansas City, MO 64153
816-891-7700
(M-F, 8:00-4:00 Central Time)
0575-D1109
NET CONTENTS:
LOT NO.:
EXP DATE:
| SODIUM IODIDE-IODINE COMPLEX
iodine concentrate |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - WestAgro, Inc. (069288728) |
| Registrant - WestAgro, Inc. (069288728) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| WestAgro, Inc. | 147528723 | analysis, label, manufacture, pack, api manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| K Klean dba WestAgro, Inc. | 002222368 | analysis, label, manufacture, pack, api manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| WestAgro, Inc. | 044610140 | analysis, label, manufacture, pack, api manufacture | |