Label: ZEPOL TOPICAL ANALGESIC- camphor, menthol ointment
- NDC Code(s): 55715-001-01, 55715-001-12, 55715-001-90
- Packager: Laboratorios Zepol S.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
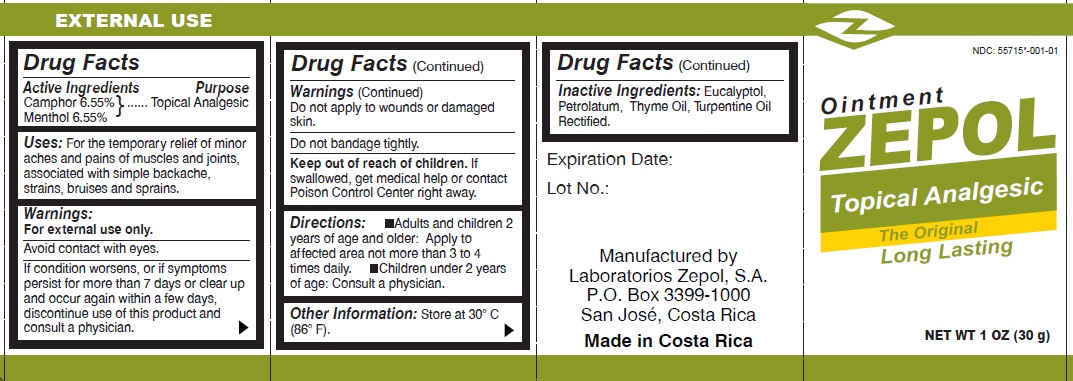
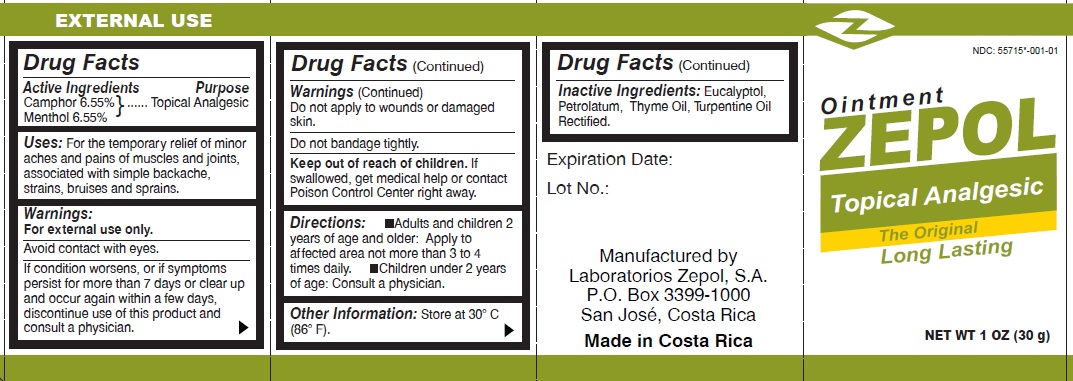
- Drug Facts
- Active Ingredients
- Uses
- Warnings
- Directions:
- Other information
- Inactive Ingredients
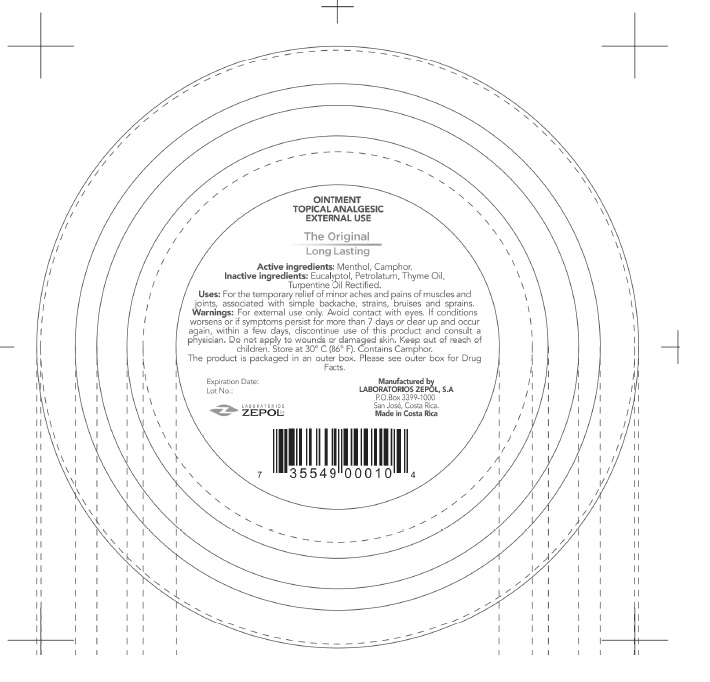
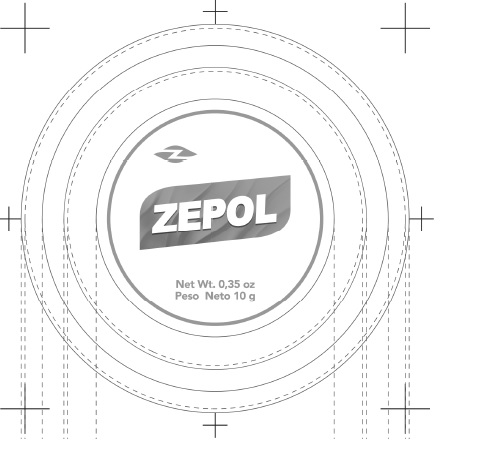
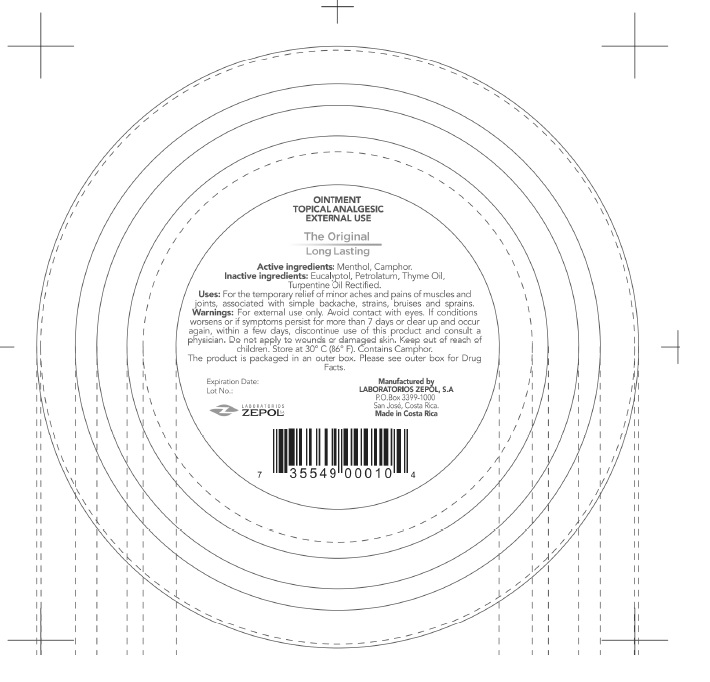
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
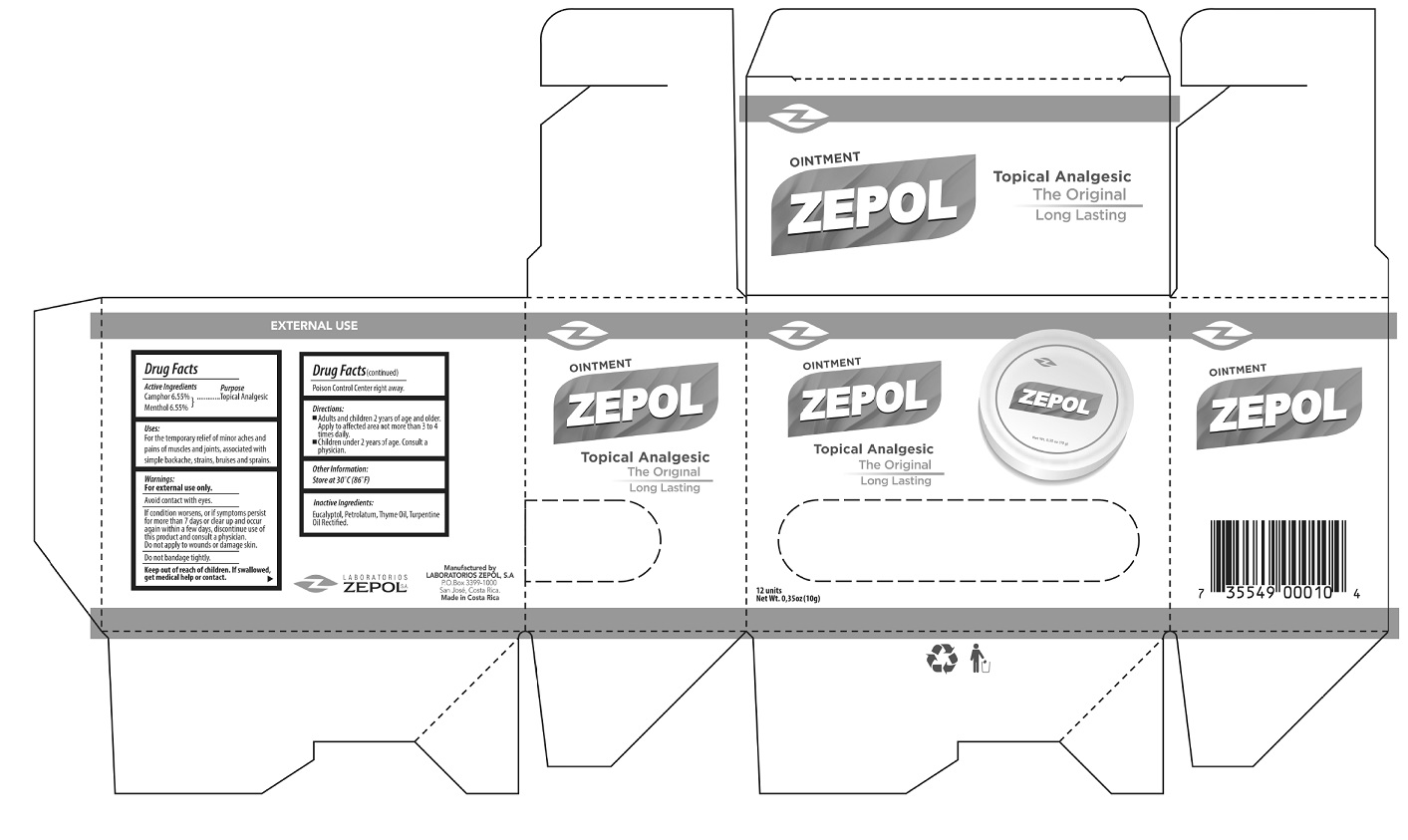
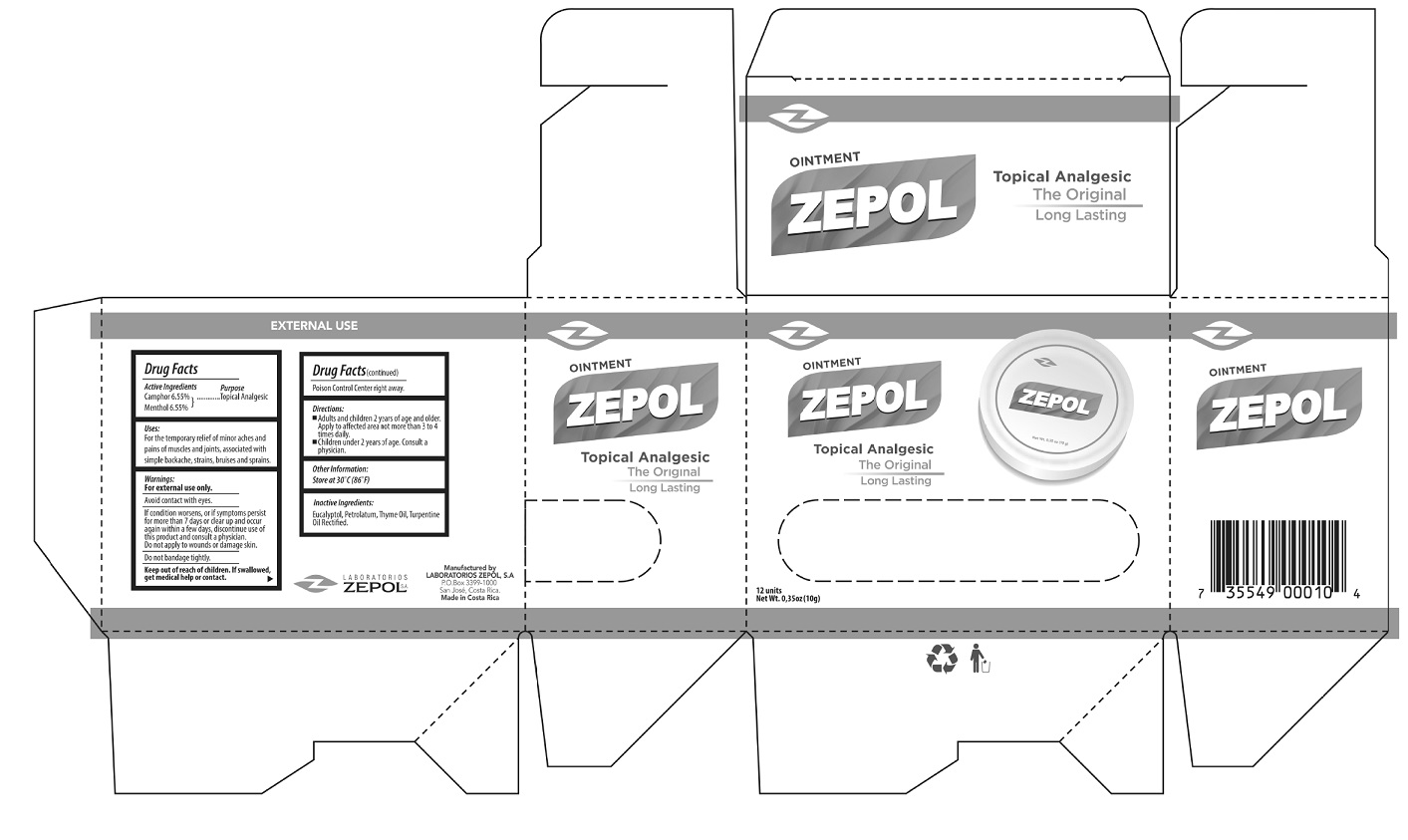
- Package Labeling:
- Package Labeling:
- Zepol Topical Analgesic Ointment,12 units (0.35oz/10g) (55715-001-12)
-
INGREDIENTS AND APPEARANCE
ZEPOL TOPICAL ANALGESIC
camphor, menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55715-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 6.55 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6.55 g in 100 g Inactive Ingredients Ingredient Name Strength EUCALYPTOL (UNII: RV6J6604TK) PETROLATUM (UNII: 4T6H12BN9U) TURPENTINE (UNII: XJ6RUH0O4G) THYME OIL (UNII: 2UK410MY6B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55715-001-01 30 g in 1 TUBE; Type 0: Not a Combination Product 10/05/2009 2 NDC:55715-001-90 1 in 1 BOX 10/05/2009 2 90 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:55715-001-12 12 in 1 BOX 10/01/2019 3 10 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/05/2009 Labeler - Laboratorios Zepol S.A. (853070985) Establishment Name Address ID/FEI Business Operations Laboratorios Zepol S.A 853070985 manufacture(55715-001)