SIMVASTATIN- simvastatin tablet
REMEDYREPACK INC.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use simvastatin safely and effectively. See full prescribing information for simvastatin tablets.
Simvastatin Tablets, USP
Initial U.S. Approval: 1991
SPL INDEXING DATA ELEMENTS
These highlights do not include all the information needed to use simvastatin safely and effectively. See full prescribing information for simvastatin tablets.
Simvastatin Tablets, USP
Initial U.S. Approval: 1991
RECENT MAJOR CHANGES
Dosage and Administration
Chinese Patients Taking Lipid-Modifying Doses (1 g/day Niacin) of Niacin-Containing Products
(2.5)
03/2010
Coadministration with Other Drugs
(2.6)
03/2010
Warnings and Precautions
Myopathy/Rhabdomyolysis
(5.1)
03/2010
INDICATIONS AND USAGE
Simvastatin tablets are an HMG-CoA reductase inhibitor (statin) indicated as an adjunctive therapy to diet to:
- Reduce the risk of total mortality by reducing CHD deaths and reduce the risk of non-fatal myocardial infarction, stroke, and the need for revascularization procedures in patients at high risk of coronary events. (1.1)
- Reduce elevated total-C, LDL-C, Apo B, TG and increase HDL-C in patients with primary hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia. (1.2)
- Reduce elevated TG in patients with hypertriglyceridemia and reduce TG and VLDL-C in patients with primary dysbeta (1.2)
- Reduce total-C and LDL-C in adult patients with homozygous familial hypercholesterolemia. (1.2)
- Reduce elevated total-C, LDL-C, and Apo B in boys and postmenarchal girls, 10 to 17 years of age with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy. (1.2, 1.3)
Limitations of Use
Simvastatin tablets have not been studied in Fredrickson Types I and V dyslipidemias.
(1.4)
DOSAGE AND ADMINISTRATION
Dose range is 5 to 80 mg/day.
(2.1)
- Recommended usual starting dose is 20 to 40 mg once a day in the evening. (2.1)
- Recommended starting dose for patients at high risk of CHD is 40 mg/day. (2.1)
- Adolescents (10 to 17 years of age) with HeFH: starting dose is 10 mg/day; maximum recommended dose is 40 mg/day. (2.3)
- DOSAGE FORMS AND STRENGTHS
(3)
CONTRAINDICATIONS
Hypersensitivity to any component of this medication.
(4
,
6.2)
- Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels. (4, 5.2)
- Women who are pregnant or may become pregnant. (4, 8.1)
- Nursing mothers. (4, 8.3)
- WARNINGS AND PRECAUTIONS
Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase with higher doses and concomitant use of certain CYP3A4 inhibitors, gemfibrozil, cyclosporine, danazol, amiodarone, verapamil, and diltiazem. Predisposing factors include advanced age (uncontrolled hypothyroidism, and renal impairment.
(5.1
,
8.5
,
8.6)
- Patients should be advised to report promptly any symptoms of myopathy. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. See Drug Interaction table. (5.1)
- Liver enzyme abnormalities and monitoring: Persistent elevations in hepatic transaminase can occur. Monitor liver enzymes before and during treatment. Patients titrated to the 80 mg dose should receive more frequent liver function tests than patients on lower doses. (5.2)
- ADVERSE REACTIONS
Most common adverse reactions (incidenceare: upper respiratory infection, headache, abdominal pain, constipation, and nausea.
(6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis (
2.6
,
5.1
,
7.1
,
7.2
,
7.3
,
7.4)
Interacting AgentsPrescribing Recommendations
Itraconazole, ketoconazole, erythromycin,Avoid simvastatinclarithromycin, telithromycin, HIV proteaseinhibitors, nefazodoneGemfibrozil, cyclosporine, danazolDo not exceed 10 mg simvastatin dailyAmiodarone, verapamilDo not exceed 20 mg simvastatin dailyDiltiazemDo not exceed 40 mg simvastatin dailyGrapefruit juiceAvoid large quantities of grapefruit juice (>1 quart daily) Coumarin anticoagulants: Concomitant use with simvastatin prolongs INR. Achieve stable INR prior to starting simvastatin. Monitor INR frequently until stable upon initiation or alteration of simvastatin therapy.
(7.7)
- USE IN SPECIFIC POPULATIONS
Severe renal impairment: patients should be started at 5 mg/day and be closely monitored.
(2.4
,
8.6)
See
17for PATIENT COUNSELING INFORMATION
SPL PATIENT PACKAGE INSERT
FULL PRESCRIBING INFORMATION: CONTENTS
*
1 INDICATIONS AND USAGE
1.1 Reductions in Risk of CHD Mortality and Cardiovascular Events
1.2 Hyperlipidemia
1.3 Adolescent Patients with Heterozygous Familial Hypercholesterolemia (HeFH)
1.4 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Patients with Homozygous Familial Hypercholesterolemia
2.3 Adolescents (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia
2.4 Patients with Renal Impairment
2.5 Chinese Patients Taking Lipid-Modifying Doses (g/day Niacin) of Niacin-Containing Products
2.6 Coadministration with Other Drugs
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy/Rhabdomyolysis
5.2 Liver Dysfunction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Interactions
7.2 Lipid-Lowering Drugs That Can Cause Myopathy When Given Alone
7.3 Cyclosporine or Danazol
7.4 Amiodarone, Verapamil, or Diltiazem
7.5 Niacin
7.6 Digoxin
7.7 Coumarin Anticoagulants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Clinical Studies in Adults
14.2 Clinical Studies in Adolescents
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Muscle Pain
17.2 Liver Enzymes
17.3 Pregnancy
17.4 Breastfeeding
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (30 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg Bulk Tablet Label
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (30 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg Bulk Tablet Label
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (30 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg Bulk Tablet Label
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg (30 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg Bulk Tablet Label
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 80 mg (30 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 80 mg Bulk Tablet Label
*Sections or subsections omitted from the full prescribing information are not listed
INDICATIONS & USAGE
Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In patients with coronary heart disease (CHD) or at high risk of CHD, simvastatin tablets can be started simultaneously with diet.
1.1 Reductions in Risk of CHD Mortality and Cardiovascular Events
In patients at high risk of coronary events because of existing coronary heart disease, diabetes, peripheral vessel disease, history of stroke or other cerebrovascular disease, simvastatin tablets are indicated to:
1.2 Hyperlipidemia
Simvastatin tablets are indicated to:
Reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), and triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hyperlipidemia (Fredrickson type IIa, heterozygous familial and nonfamilial) or mixed dyslipidemia (Fredrickson type IIb).
1.3 Adolescent Patients with Heterozygous Familial Hypercholesterolemia (HeFH)
Simvastatin tablets are indicated as an adjunct to diet to reduce total-C, LDL-C, and Apo B levels in adolescent boys and girls who are at least one year post-menarche, 10 to 17 years of age, with HeFH, if after an adequate trial of diet therapy the following findings are present:
1. LDL cholesterol remains
2. LDL cholesterol remainsmg/dL and
There is a positive family history of premature cardiovascular disease (CVD) or
1.4 Limitations of Use
Simvastatin tablets have not been studied in conditions where the major abnormality is elevation of chylomicrons (i.e., hyperlipidemia Fredrickson types I and V).
DOSAGE & ADMINISTRATION
2.1 Recommended Dosing
The dosage range is 5 to 80 mg/day. In patients with CHD or at high risk of CHD, simvastatin tablets can be started simultaneously with diet. The recommended usual starting dose is 20 to 40 mg once a day in the evening. For patients at high risk for a CHD event due to existing CHD, diabetes, peripheral vessel disease, history of stroke or other cerebrovascular disease, the recommended starting dose is 40 mg/day. Lipid determinations should be performed after 4 weeks of therapy and periodically thereafter.
2.2 Patients with Homozygous Familial Hypercholesterolemia
The recommended dosage is 40 mg/day in the evening or 80 mg/day in 3 divided doses of 20 mg, 20 mg, and an evening dose of 40 mg. Simvastatin tablets should be used as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) in these patients or if such treatments are unavailable.
2.3 Adolescents (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia
Clinical Studies (14.2)
]. Adjustments should be made at intervals of 4 weeks or more.
1 National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 89(3):495-501. 1992.
2.4 Patients with Renal Impairment
Warnings and Precautions (5.1)
Clinical Pharmacology (12.3)
].
2.5 Chinese Patients Taking Lipid-Modifying Doses (g/day Niacin) of Niacin-Containing Products
Because of an increased risk for myopathy, caution should be used when treating Chinese patients with simvastatin coadministered with lipid-modifying doses (g/day niacin) of niacin-containing products. Because the risk for myopathy is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products. The cause of the increased risk of myopathy is not known. It is also unknown if the risk for myopathy with coadministration of simvastatin with lipid-modifying doses of niacin-containing products observed in Chinese patients applies to other Asian patients. [See
Warnings and Precautions (5.1)
.]
2.6 Coadministration with Other Drugs
Concomitant Lipid-Lowering Therapy
- Simvastatin tablets may be used concomitantly with bile acid sequestrants.
- Combination therapy with gemfibrozil increases simvastatin exposure. Therefore, if simvastatin tablets are used in combination with gemfibrozil, the dose of simvastatin tablets should not exceed 10 mg/day [see Warnings and Precautions (5.1), Drug Interactions (7.2), and Clinical Pharmacology (12.3)].
Patients taking Cyclosporine or Danazol
Simvastatin tablets therapy should begin with 5 mg/day and should not exceed 10 mg/day [see
Warnings and Precautions (5.1)
and
Drug Interactions (7.3)
Patients taking Amiodarone or Verapamil
The dose of simvastatin tablets should not exceed 20 mg/day [see
Warnings and Precautions (5.1)
,
Drug Interactions (7.4)
, and
Clinical Pharmacology (12.3)
- Patients taking Diltiazem
The dose of simvastatin tablets should not exceed 40 mg/day [see
Warnings and Precautions (5.1)
,
Drug Interactions (7.4)
, and
Clinical Pharmacology (12.3)
DOSAGE FORMS & STRENGTHS
- Tablets simvastatin 5 mg are yellow colored, round shaped, biconvex, film coated tablets, debossed withA'on one side and15'on the other side.
- Tablets simvastatin 10 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed withA'on one side and01'on the other side.
- Tablets simvastatin 20 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed withA'on one side and02'on the other side.
- Tablets simvastatin 40 mg are pink colored, round shaped, biconvex, film coated tablets, debossed withA'on one side and03'on the other side.
- Tablets simvastatin 80 mg are pink colored, capsule shaped, biconvex, film coated tablets, debossed withA'on one side and04'on the other side.
CONTRAINDICATIONS
Simvastatin tablets are contraindicated in the following conditions:
- Hypersensitivity to any component of this medication [see Adverse Reactions (6.2)].
- Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels [see Warnings and Precautions (5.2)].
- Women who are pregnant or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Because HMG-CoA reductase inhibitors (statins) decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, simvastatin tablets may cause fetal harm when administered to a pregnant woman. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. There are no adequate and well-controlled studies of use with simvastatin tablets during pregnancy; however, in rare reports congenital anomalies were observed following intrauterine exposure to statins. In rat and rabbit animal reproduction studies, simvastatin revealed no evidence of teratogenicity. Simvastatin tablets should be administered to women of childbearing age only when such patients are highly unlikely to conceive. If the patient becomes pregnant while taking this drug, simvastatin tablets should be discontinued immediately and the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
- Nursing mothers. It is not known whether simvastatin is excreted into human milk; however, a small amount of another drug in this class does pass into breast milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require treatment with simvastatin tablets should not breastfeed their infants [see Use in Specific Populations (8.3)].
WARNINGS AND PRECAUTIONS
.1 Myopathy/Rhabdomyolysis
Simvastatin, like other statins, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by high levels of statin activity in plasma. Predisposing factors for myopathy include advanced age (years), uncontrolled hypothyroidism, and renal impairment.
As with other statins, the risk of myopathy/rhabdomyolysis is dose related. In a clinical trial database in which 41,050 patients were treated with simvastatin with 24,747 (approximately 60%) treated for at least 4 years, the incidence of myopathy was approximately 0.02%, 0.08% and 0.53% at 20, 40 and 80 mg/day, respectively. In these trials, patients were carefully monitored and some interacting medicinal products were excluded.
All patients starting therapy with simvastatin, or whose dose of simvastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with simvastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.
Many of the patients who have developed rhabdomyolysis on therapy with simvastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients merit closer monitoring. Therapy with simvastatin should be temporarily stopped a few days prior to elective major surgery and when any major medical or surgical condition supervenes.
Drug Interactions
The risk of myopathy and rhabdomyolysis is increased by high levels of statin activity in plasma. Simvastatin is metabolized by the cytochrome P450 isoform 3A4. Certain drugs which inhibit this metabolic pathway can raise the plasma levels of simvastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, and other antifungal azoles, the macrolide antibiotics erythromycin and clarithromycin, and the ketolide antibiotic telithromycin, HIV protease inhibitors, the antidepressant nefazodone, or large quantities of grapefruit juice (>1 quart daily). The use of simvastatin concomitantly with these CYP3A4 inhibitors should be avoided. If treatment with itraconazole, ketoconazole, erythromycin, clarithromycin or telithromycin is unavoidable, therapy with simvastatin should be suspended during the course of treatment. [See
Drug Interactions (7)
.]
The benefits of the combined use of simvastatin with the following drugs should be carefully weighed against the potential risks of combinations: gemfibrozil, other lipid-lowering drugs (other fibrates org/day of niacin), cyclosporine, danazol, amiodarone, verapamil, or diltiazem.
Caution should be used when prescribing other fibrates with simvastatin, as these agents can cause myopathy when given alone.
Cases of myopathy/rhabdomyolysis have been observed with simvastatin coadministered with lipid-modifying doses (g/day niacin) of niacin-containing products. In an ongoing, double-blind, randomized cardiovascular outcomes trial, an independent safety monitoring committee identified that the incidence of myopathy is higher in Chinese compared with non-Chinese patients taking simvastatin 40 mg coadministered with lipid-modifying doses of a niacin-containing product. Because the risk for myopathy is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products. It is unknown if the risk for myopathy with coadministration of simvastatin with lipid-modifying doses of niacin-containing products observed in Chinese patients applies to other Asian patients.
Prescribing recommendations for interacting agents are summarized in Table 1 [see also
Dosage and Administration (2.6)
,
Drug Interactions (7)
,
Clinical Pharmacology (12.3)
].
TABLE 1 Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis
Interacting AgentsPrescribing RecommendationsItraconazoleAvoid simvastatinKetoconazoleErythromycinClarithromycinTelithromycinHIV Protease inhibitorsNefazodoneGemfibrozil*Do not exceed 10 mg simvastatin dailyCyclosporine+Danazol+AmiodaroneDo not exceed 20 mg simvastatin dailyVerapamilDiltiazemDo not exceed 40 mg simvastatin dailyGrapefruit juiceAvoid large quantities of grapefruit juice (>1 quart daily)* The combined use of simvastatin with gemfibrozil should be avoided, unless the benefits are likely to outweigh the increased risks of this drug combination.
The benefits of the use of simvastatin in patients receiving cyclosporine or danazol should be carefully weighed against the risks of these combinations.
The combined use of simvastatin at doses higher than 20 mg daily with amiodarone or verapamil should be avoided unless the clinical benefit is likely to outweigh the increased risk of myopathy.
The combined use of simvastatin in patients receiving diltiazem should not exceed 40 mg daily unless the clinical benefit is likely to outweigh the increased risk of myopathy.
5.2 Liver Dysfunction
Persistent increases (to more than 3X the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies. When drug treatment was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity.
In the Scandinavian Simvastatin Survival Study (4S) [see
Clinical Studies (14.1)
In 2 controlled clinical studies in 1,105 patients, the 12-month incidence of persistent hepatic transaminase elevation without regard to drug relationship was 0.9% and 2.1% at the 40 and 80 mg dose, respectively. No patients developed persistent liver function abnormalities following the initial 6 months of treatment at a given dose.
It is recommended that liver function tests be performed before the initiation of treatment, and thereafter when clinically indicated. Patients titrated to the 80 mg dose should receive an additional test prior to titration, 3 months after titration to the 80 mg dose, and periodically thereafter (e.g., semiannually) for the first year of treatment. Patients who develop increased transaminase levels should be monitored with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality(ies) return to normal. Should an increase in AST or ALT of 3X ULN or greater persist, withdrawal of therapy with simvastatin is recommended.
The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained transaminase elevations are contraindications to the use of simvastatin.
As with other lipid-lowering agents, moderate (less than 3X ULN) elevations of serum transaminases have been reported following therapy with simvastatin. These changes appeared soon after initiation of therapy with simvastatin, were often transient, were not accompanied by any symptoms and did not require interruption of treatment.
ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In the pre-marketing controlled clinical studies and their open extensions (2,423 patients with median duration of follow-up of approximately 18 months), 1.4% of patients were discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: gastrointestinal disorders (0.5%), myalgia (0.1%), and arthralgia (0.1%). The most commonly reported adverse reactions (incidencein simvastatin controlled clinical trials were: upper respiratory infections (9%), headache (7.4%), abdominal pain (7.3%), constipation (6.6%), and nausea (5.4%).
Scandinavian Simvastatin Survival Study
In 4S involving 4,444 (age range 35 to 71 years, 19% women, 100% Caucasians) treated with 20 to 40 mg/day of simvastatin (n=2,221) or placebo (n=2,223) over a median of 5.4 years, adverse reactions reported inof patients and at a rate greater than placebo are shown in Table 2.
TABLE 2 Adverse Reactions Reported Regardless of Causality byof Patients Treated with Simvastatin and Greater than Placebo in 4S
SimvastatinPlacebo (N = 2,223) %(N=2,221)(N=2,223)%%Body as a WholeEdema/ swelling2.72.3Abdominal pain5.95.8Cardiovascular System DisordersAtrial fibrillation5.75.1Digestive System DisordersConstipation2.21.6Gastritis4.93.9Endocrine DisordersDiabetes mellitus4.23.6Musculoskeletal DisordersMyalgia3.73.2Nervous System / Psychiatric DisordersHeadache2.52.1Insomnia43.8Vertigo4.54.2Respiratory System DisordersBronchitis6.66.3Sinusitis2.31.8Skin / Skin Appendage DisordersEczema4.53Urogenital System DisordersInfection, urinary tract3.23.1
Heart Protection Study
In the Heart Protection Study (HPS), involving 20,536 patients (age range 40 to 80 years, 25% women, 97% Caucasians, 3% other races) treated with simvastatin 40 mg/day (n=10,269) or placebo (n=10,267) over a mean of 5 years, only serious adverse reactions and discontinuations due to any adverse reactions were recorded. Discontinuation rates due to adverse reactions were 4.8% in patients treated with simvastatin compared with 5.1% in patients treated with placebo. The incidence of myopathy/rhabdomyolysis was <0.1% in patients treated with simvastatin.
Other Clinical Studies
Other adverse reactions reported in clinical trials were: diarrhea, rash, dyspepsia, flatulence, and asthenia.
Laboratory Tests
Marked persistent increases of hepatic transaminases have been noted [see
Warnings and Precautions (5.2)
]. Elevated alkaline phosphatase andtranspeptidase have also been reported. About 5% of patients had elevations of CK levels of 3 or more times the normal value on one or more occasions. This was attributable to the noncardiac fraction of CK. [See
Warnings and Precautions (5.1)
.]
Adolescent Patients (ages 10 to 17 years)
In a 48-week, controlled study in adolescent boys and girls who were at least 1 year post-menarche, 10 to 17 years of age (43.4% female, 97.7% Caucasians, 1.7% Hispanics, 0.6% Multiracial) with heterozygous familial hypercholesterolemia (n=175), treated with placebo or simvastatin (10 to 40 mg daily), the most common adverse reactions observed in both groups were upper respiratory infection, headache, abdominal pain, and nausea [see
Use in Specific Populations (8.4)
and
Clinical Studies (14.2)
6.2 Post-Marketing Experience
Because the below reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following additional adverse reactions have been identified during postapproval use of simvastatin: pruritus, alopecia, a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails), dizziness, muscle cramps, myalgia, pancreatitis, memory impairment, paresthesia, peripheral neuropathy, vomiting and anemia, rhabdomyolysis, hepatitis/jaundice, hepatic failure, depression.
An apparent hypersensitivity syndrome has been reported rarely which has included some of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
DRUG INTERACTIONS
7.1 CYP3A4 Interactions
The risk of myopathy is increased by reducing the elimination of simvastatin. Hence when simvastatin is used with an inhibitor of CYP3A4 (e.g., as listed below), elevated plasma levels of HMG-CoA reductase inhibitory activity can increase the risk of myopathy and rhabdomyolysis, particularly with higher doses of simvastatin. [See
Warnings and Precautions (5.1)
and
Clinical Pharmacology (12.3)
.]
Itraconazole, ketoconazole, and other antifungal azoles
Macrolide antibiotics erythromycin, clarithromycin, and the ketolide antibiotic telithromycin
HIV protease inhibitors
Antidepressant nefazodone
Grapefruit juice in large quantities (>1 quart daily)
Concomitant use of these drugs and any medication labeled as having a strong inhibitory effect on CYP3A4 should be avoided unless the benefits of combined therapy outweigh the increased risk. If treatment with itraconazole, ketoconazole, erythromycin, clarithromycin or telithromycin is unavoidable, therapy with simvastatin should be suspended during the course of treatment.
7.2 Lipid-Lowering Drugs That Can Cause Myopathy When Given Alone
The risk of myopathy is increased by gemfibrozil [see
Dosage and Administration (2.6)
] and to a lesser extent by other fibrates [see
Warnings and Precautions (5.1)
7.3 Cyclosporine or Danazol
The risk of myopathy/rhabdomyolysis is increased by concomitant administration of cyclosporine or danazol particularly with higher doses of simvastatin [see
Warnings and Precautions (5.1)
and
Clinical Pharmacology (12.3)
7.4 Amiodarone, Verapamil, or Diltiazem
The risk of myopathy/rhabdomyolysis is increased by concomitant administration of amiodarone, verapamil, or diltiazem with higher doses of simvastatin [see
Warnings and Precautions (5.1)
].
7.5 Niacin
Cases of myopathy/rhabdomyolysis have been observed with simvastatin coadministered with lipid-modifying doses (g/day niacin) of niacin-containing products. In particular, caution should be used when treating Chinese patients with simvastatin coadministered with lipid-modifying doses of niacin-containing products. Because the risk for myopathy is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products. [See
Warnings and Precautions (5.1)
and
Clinical Pharmacology (12.3)
.]
7.6 Digoxin
In one study, concomitant administration of digoxin with simvastatin resulted in a slight elevation in digoxin concentrations in plasma. Patients taking digoxin should be monitored appropriately when simvastatin is initiated [see
Clinical Pharmacology (12.3)
7.7 Coumarin Anticoagulants
In two clinical studies, one in normal volunteers and the other in hypercholesterolemic patients, simvastatin 20 to 40 mg/day modestly potentiated the effect of coumarin anticoagulants: the prothrombin time, reported as International Normalized Ratio (INR), increased from a baseline of 1.7 to 1.8 and from 2.6 to 3.4 in the volunteer and patient studies, respectively. With other statins, clinically evident bleeding and/or increased prothrombin time has been reported in a few patients taking coumarin anticoagulants concomitantly. In such patients, prothrombin time should be determined before starting simvastatin and frequently enough during early therapy to ensure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of simvastatin is changed or discontinued, the same procedure should be repeated. Simvastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X [See
Contraindications (4)
.]
There are rare reports of congenital anomalies following intrauterine exposure to statins. In a review2 of approximately 100 prospectively followed pregnancies in women exposed to simvastatin or another structurally related statin, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed those expected in the general population. However, the study was only able to exclude a 3- to 4-fold increased risk of congenital anomalies over the background rate. In 89% of these cases, drug treatment was initiated prior to pregnancy and was discontinued during the first trimester when pregnancy was identified.
Simvastatin was not teratogenic in rats or rabbits at doses (25, 10 mg/kg/day, respectively) that resulted in 3 times the human exposure based on mg/m2 surface area. However, in studies with another structurally-related statin, skeletal malformations were observed in rats and mice.
Women of childbearing potential, who require treatment with simvastatin for a lipid disorder, should be advised to use effective contraception. For women trying to conceive, discontinuation of simvastatin should be considered. If pregnancy occurs, simvastatin should be immediately discontinued.
2 Manson, J.M., Freyssinges, C., Ducrocq, M.B., Stephenson, W.P., Postmarketing Surveillance of Lovastatin and Simvastatin Exposure During Pregnancy, Reproductive Toxicology, 10(6):439-446, 1996.
8.3 Nursing Mothers
It is not known whether simvastatin is excreted in human milk. Because a small amount of another drug in this class is excreted in human milk and because of the potential for serious adverse reactions in nursing infants, women taking simvastatin should not nurse their infants. A decision should be made whether to discontinue nursing or discontinue drug, taking into account the importance of the drug to the mother [see
Contraindications (4)
].
8.4 Pediatric Use
Safety and effectiveness of simvastatin in patients 10 to 17 years of age with heterozygous familial hypercholesterolemia have been evaluated in a controlled clinical trial in adolescent boys and in girls who were at least 1 year post-menarche. Patients treated with simvastatin had an adverse reaction profile similar to that of patients treated with placebo. Doses greater than 40 mg have not been studied in this population. In this limited controlled study, there was no significant effect on growth or sexual maturation in the adolescent boys or girls, or on menstrual cycle length in girls. [See
Dosage and Administration (2.3)
,
Adverse Reactions (6.1)
,
Clinical Studies (14.2)
.] Adolescent females should be counseled on appropriate contraceptive methods while on simvastatin therapy [see
Contraindications (4)
and
Use in Specific Populations (8.1)
]. Simvastatin has not been studied in patients younger than 10 years of age, nor in pre-menarchal girls.
8.5 Geriatric Use
Of the 2,423 patients who received simvastatin in Phase III clinical studies and the 10,269 patients in the Heart Protection Study who received simvastatin, 363 (15%) and 5,366 (52%), respectively wereyears old. In HPS, 615 (6%) wereyears old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Since advanced age (years) is a predisposing factor for myopathy, simvastatin should be prescribed with caution in the elderly. [See
Clinical Pharmacology (12.3)
.]
A pharmacokinetic study with simvastatin showed the mean plasma level of statin activity to be approximately 45% higher in elderly patients between 70 to 78 years of age compared with patients between 18 to 30 years of age. In 4S, 1,021 (23%) of 4,444 patients were 65 or older. Lipid-lowering efficacy was at least as great in elderly patients compared with younger patients, and simvastatin significantly reduced total mortality and CHD mortality in elderly patients with a history of CHD. In HPS, 52% of patients were elderly (4,891 patients 65 to 69 years and 5,806 patients 70 years or older). The relative risk reductions of CHD death, non-fatal MI, coronary and non-coronary revascularization procedures, and stroke were similar in older and younger patients [see
Clinical Studies (14.1)
8.6 Renal Impairment
Caution should be exercised when simvastatin is administered to patients with severe renal impairment. [SeeDosage and Administration (2.4)
8.7 Hepatic Impairment
Simvastatin is contraindicated in patients with active liver disease which may include unexplained persistent elevations in hepatic transaminase levels [see
Contraindications (4)
and
Warnings and Precautions (5.2)
].
OVERDOSAGE
Significant lethality was observed in mice after a single oral dose of 9 g/m2. No evidence of lethality was observed in rats or dogs treated with doses of 30 and 100 g/m2, respectively. No specific diagnostic signs were observed in rodents. At these doses the only signs seen in dogs were emesis and mucoid stools.
DESCRIPTION
Simvastatin is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. After oral ingestion, simvastatin, which is an inactive lactone, is hydrolyzed to the correspondingform. This is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, which is an early and rate-limiting step in the biosynthesis of cholesterol.
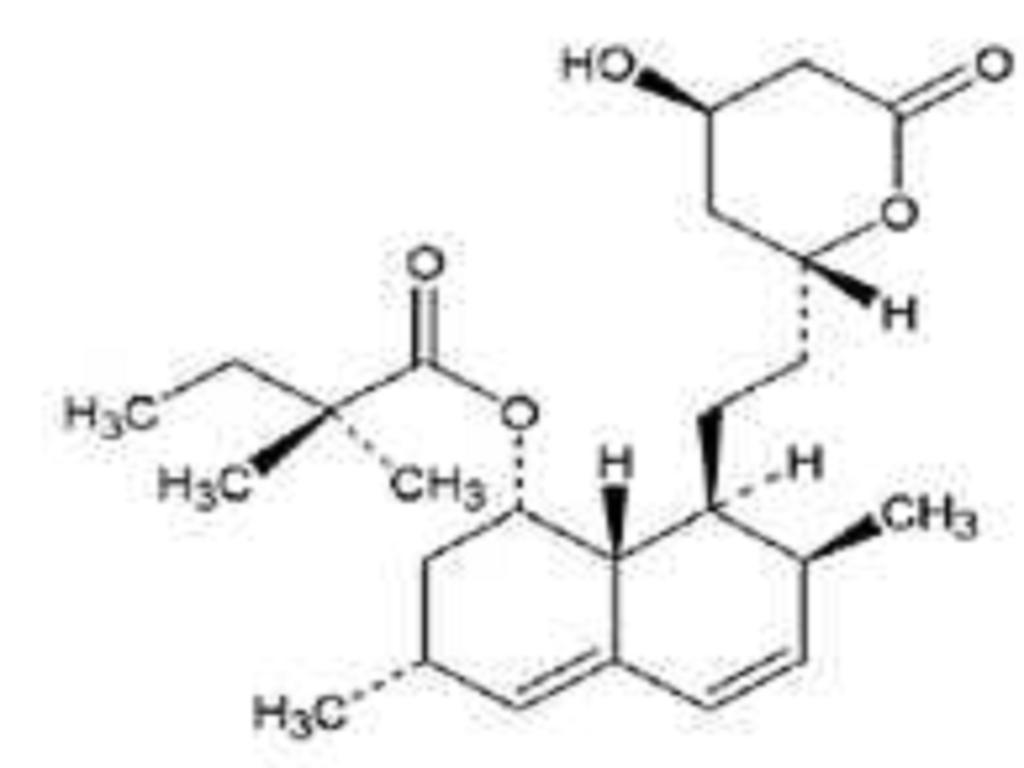
Simvastatin is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1The molecular formula of simvastatin is C25H38O5 and its molecular weight is 418.57. Its structural formula is:
Simvastatin is a white to off-white, nonhygroscopic, crystalline powder that is practically insoluble in water, and freely soluble in chloroform, methanol and ethanol.
Tablets simvastatin for oral administration contain either 5 mg, 10 mg, 20 mg, 40 mg or 80 mg of simvastatin and the following inactive ingredients: ascorbic acid, lactose monohydrate, microcrystalline cellulose, pregelatinized starch, hydroxypropyl cellulose, hypromellose, titanium dioxide, talc, citric acid monohydrate, isopropyl alcohol, magnesium stearate and butylated hydroxyanisole. Simvastatin 5 mg also contains ferric oxide yellow, simvastatin 10 mg and simvastatin 20 mg also contains ferric oxide red and ferric oxide yellow, simvastatin 40 mg and simvastatin 80 mg also contains ferric oxide red.
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Simvastatin is a prodrug and is hydrolyzed to its activeform, simvastatin acid, after administration. Simvastatin is a specific inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in the biosynthetic pathway for cholesterol. In addition, simvastatin reduces VLDL and TG and increases HDL-C.
PHARMACODYNAMICS
12.2 Pharmacodynamics
Epidemiological studies have demonstrated that elevated levels of total-C, LDL-C, as well as decreased levels of HDL-C are associated with the development of atherosclerosis and increased cardiovascular risk. Lowering LDL-C decreases this risk. However, the independent effect of raising HDL-C or lowering TG on the risk of coronary and cardiovascular morbidity and mortality has not been determined.
PHARMACOKINETICS
12.3 Pharmacokinetics
Simvastatin is a lactone that is readily hydrolyzed in vivo to the correspondinga potent inhibitor of HMG-CoA reductase. Inhibition of HMG-CoA reductase is the basis for an assay in pharmacokinetic studies of themetabolites (active inhibitors) and, following base hydrolysis, active plus latent inhibitors (total inhibitors) in plasma following administration of simvastatin.
Following an oral dose of 14C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Plasma concentrations of total radioactivity (simvastatin plus 14C-metabolites) peaked at 4 hours and declined rapidly to about 10% of peak by 12 hours postdose. Since simvastatin undergoes extensive first-pass extraction in the liver, the availability of the drug to the general circulation is low (<5%).
Both simvastatin and itsmetabolite are highly bound (approximately 95%) to human plasma proteins. Rat studies indicate that when radiolabeled simvastatin was administered, simvastatinradioactivity crossed the blood-brain barrier.
The major active metabolites of simvastatin present in human plasma are theof simvastatin and its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives. Peak plasma concentrations of both active and total inhibitors were attained within 1.3 to 2.4 hours postdose. While the recommended therapeutic dose range is 5 to 80 mg/day, there was no substantial deviation from linearity of AUC of inhibitors in the general circulation with an increase in dose to as high as 120 mg. Relative to the fasting state, the plasma profile of inhibitors was not affected when simvastatin was administered immediately before an American Heart Association recommended low-fat meal.
In a study including 16 elderly patients between 70 and 78 years of age who received simvastatin 40 mg/day, the mean plasma level of HMG-CoA reductase inhibitory activity was increased approximately 45% compared with 18 patients between 18 to 30 years of age. Clinical study experience in the elderly (n=1522), suggests that there were no overall differences in safety between elderly and younger patients [see
Use in Specific Populations (8.5)
].
Kinetic studies with another statin, having a similar principal route of elimination, have suggested that for a given dose level higher systemic exposure may be achieved in patients with severe renal insufficiency (as measured by creatinine clearance).
Although the mechanism is not fully understood, cyclosporine has been shown to increase the AUC of statins. The increase in AUC for simvastatin acid is presumably due, in part, to inhibition of CYP3A4.
The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma. Inhibitors of CYP3A4 can raise the plasma levels of HMG-CoA reductase inhibitory activity and increase the risk of myopathy [see
Warnings and Precautions (5.1)
and
Drug Interactions (7.1)
].
TABLE 3 Effect of Coadministered Drugs or Grapefruit Juice on Simvastatin Systemic Exposure
Geometric Mean RatioDosing of(Ratio* with / withoutCoadministeredCoadministeredcoadminstered drug)Drug orDrug or GrapefruitDosing ofNo Effect = 1Grapefruit JuiceJuiceSimvastatinAUCCmaxAvoid taking with simvastatin [see
Warnings and Precautions (5.1)
]Telithromycin200 mg QD for80 mgsimvastatin acid+ 12154 dayssimvastatin8.95.3Nelfinavir1250 mg BID for20 mg QD forsimvastatin acid14 days28 dayssimvastatin66.2Itraconazole200 mg QD for80 mgsimvastatin acid13.14 dayssimvastatin13.1Avoid >1 quart of grapefruit juice with simvastatin [see
Warnings and Precautions (5.1)
]Grapefruit Juice200 mL of double60 mg singlesimvastatin acid7(high dose)-strength TID@dosesimvastatin16Grapefruit Juice8 oz (about20 mgsimvastatin acid1.3(low dose)237mL) of single-single dosesimvistatin1.9strength#Avoid taking with >10 mg simvastatin, based on clinical and/or post-marketing experience [see
Warnings and Precautions (5.1)
]Gemfibrozil600 mg BID for40 mgsimvastatin acid2.852.18for 3 dayssimvastatin1.350.91Avoid taking with >20 mg simvastatin, based on clinical and/or post-marketing experience [see
Warnings and Precautions (5.1)
]Verapamil SR240 mg QD days80 mg onsimvastatin acid2.32.41 to 7 thenDay 10simvastatin2.52.1240 mg BID onDays 8 to 10Avoid taking with >40 mg simvastatin, based on clinical and/or post-marketing experience [see
Warnings and Precautions (5.1)
]Diltiazem120 mg BID for80 mg onsimvastatin acid2.692.6910 daysDay 10simvastatin3.12.88Diltiazem120 mg BID for20 mg onsimvastatin4.63.614 daysDay 14No dosing adjustments required for the following:Fenofibrate160 mg80 mg QDsimvastatin acid0.640.89QD x 14 daysDays 8 to 14simvastatin0.890.83Niacin extended-2 g20 mg singlesimvastatin acid1.61.84releaseIsingle dosedosesimvastatin1.41.08Amlodipine10 mg80 mg onsimvastatin acid1.581.56QD x 10 daysDay 10simvastatin1.771.47Propranolol80 mg80 mgtotal inhibitor 0.79down fromsingle dosesingle dose33.6 to 21.1ng eq/mLactive inhibitor0.79down from7 to 4.7mg eq/mL* Results based on a chemical assay except results with propranolol as indicated.
Results could be representative of the following CYP3A4 inhibitors: ketoconazole, erythromycin, clarithromycin, HIV protease inhibitors, and nefazodone.
Simvastatin acid refers to theof simvastatin.
The effect of amounts of grapefruit juice between those used in these two studies on simvastatin pharmacokinetics has not been studied.
@Double-strength: one can of frozen concentrate diluted with one can of water. Grapefruit juice was administered TID for 2 days, and 200 mL together with single dose simvastatin and 30 and 90 minutes following single dose simvastatin on Day 3.
# Single-strength: one can of frozen concentrate diluted with 3 cans of water. Grapefruit juice was administered with breakfast for 3 days, and simvastatin was administered in the evening on Day 3.
Ibecause Chinese patients have an increased risk for myopathy with simvastatin coadministered with lipid-modifying doses (1 gram/day niacin) of niacin-containing products, and the risk is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products [see
Warnings and Precautions (5.1)
and
Drug Interactions (7.5)
].
In a study of 12 healthy volunteers, simvastatin at the 80 mg dose had no effect on the metabolism of the probe cytochrome P450 isoform 3A4 (CYP3A4) substrates midazolam and erythromycin. This indicates that simvastatin is not an inhibitor of CYP3A4, and, therefore, is not expected to affect the plasma levels of other drugs metabolized by CYP3A4.
Coadministration of simvastatin (40 mg QD for 10 days) resulted in an increase in the maximum mean levels of cardioactive digoxin (given as a single 0.4 mg dose on day 10) by approximately 0.3 ng/mL.
| SIMVASTATIN
simvastatin tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |