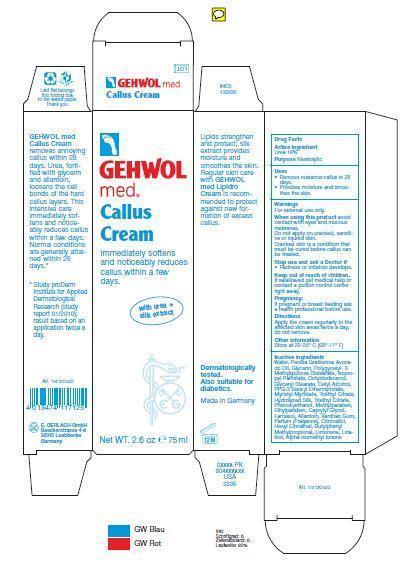
GEHWOL MED CALLUS- urea cream
Eduard Gerlach GmbH
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Inactive Ingredients
Ingredients: Aqua (Water), Persea Gratissima (Avocado) Oil, Glycerin, Polyglyceryl-3 Methylglucose Distearate, Isopropyl Palmitate, Octyldodecanol,Glyceryl Stearate, Cetyl Alcohol, PPG-3 Benzyl Ether Myristate, Myristyl Myristate, Triethyl Citrate, Hydrolyzed Silk, Allantoin, Farnesol, Xanthan Gum, Phenoxyethanol, Methylparaben, Ethylparaben, Caprylyl Glycol, Parfum (Fragrance), Citronellol, Hexyl Cinnamal, Butylphenyl Methylpropional, Limonene, Linalool, Alpha-Isomethyl Ionone
| GEHWOL MED CALLUS
urea cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Eduard Gerlach GmbH (315343350) |
| Registrant - Eduard Gerlach GmbH (315343350) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eduard Gerlach GmbH | 315343350 | manufacture(45264-006) | |
Revised: 4/2017
Document Id: e8b96d81-4f4e-44c4-80a0-ac7b75dd50cd
Set id: c91aa19a-1a04-4dd6-ab03-bd59824c1118
Version: 3
Effective Time: 20170412
Eduard Gerlach GmbH