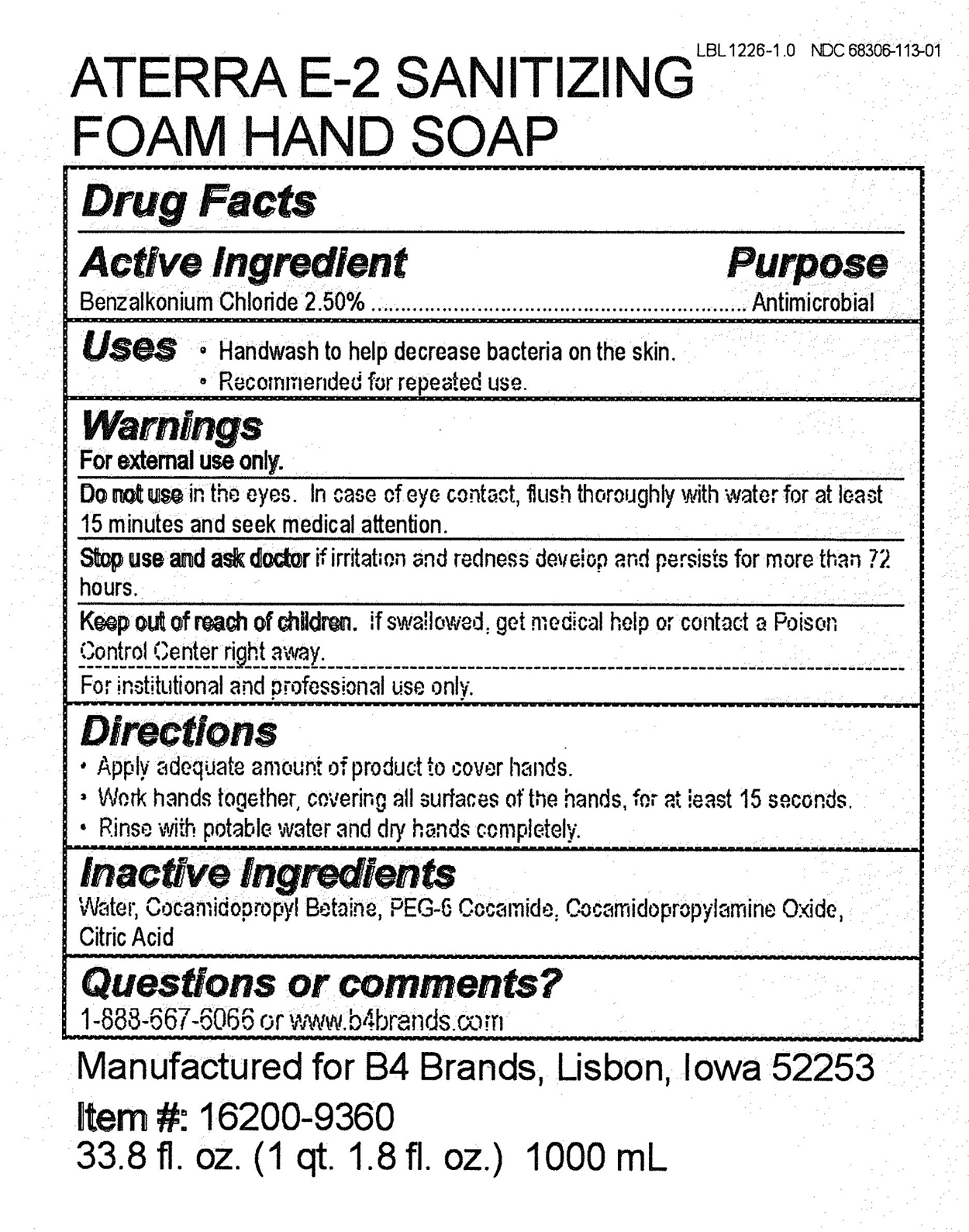
ATERRA E-2 SANITIZING FOAM HAND WASH- benzalkonium chloride soap
B4 Ventures, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aterra E-2 Sanitizing Foam Hand Soap
Warnings
For external use only.
Do not use in the eyes. In case of eye contact, flush thoroughly with water for at least 15 minutes and seek medical attention.
For institutional and professional use only.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Water, Cocamidopropyl Betaine, PEG-6 Cocamide, Cocamidopropylamine Oxide, Citric Acid
| ATERRA E-2 SANITIZING FOAM HAND WASH
benzalkonium chloride soap |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - B4 Ventures, LLC (133582853) |
| Registrant - B4 Ventures, LLC (133582853) |
Revised: 4/2019
Document Id: 860a1566-5865-ed88-e053-2991aa0ae2a4
Set id: c86420fc-1829-466f-979f-8b648ea97d7e
Version: 2
Effective Time: 20190408
B4 Ventures, LLC