FAMOTIDINE ORIGINAL STRENGTH- famotidine tablet
EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC)
----------
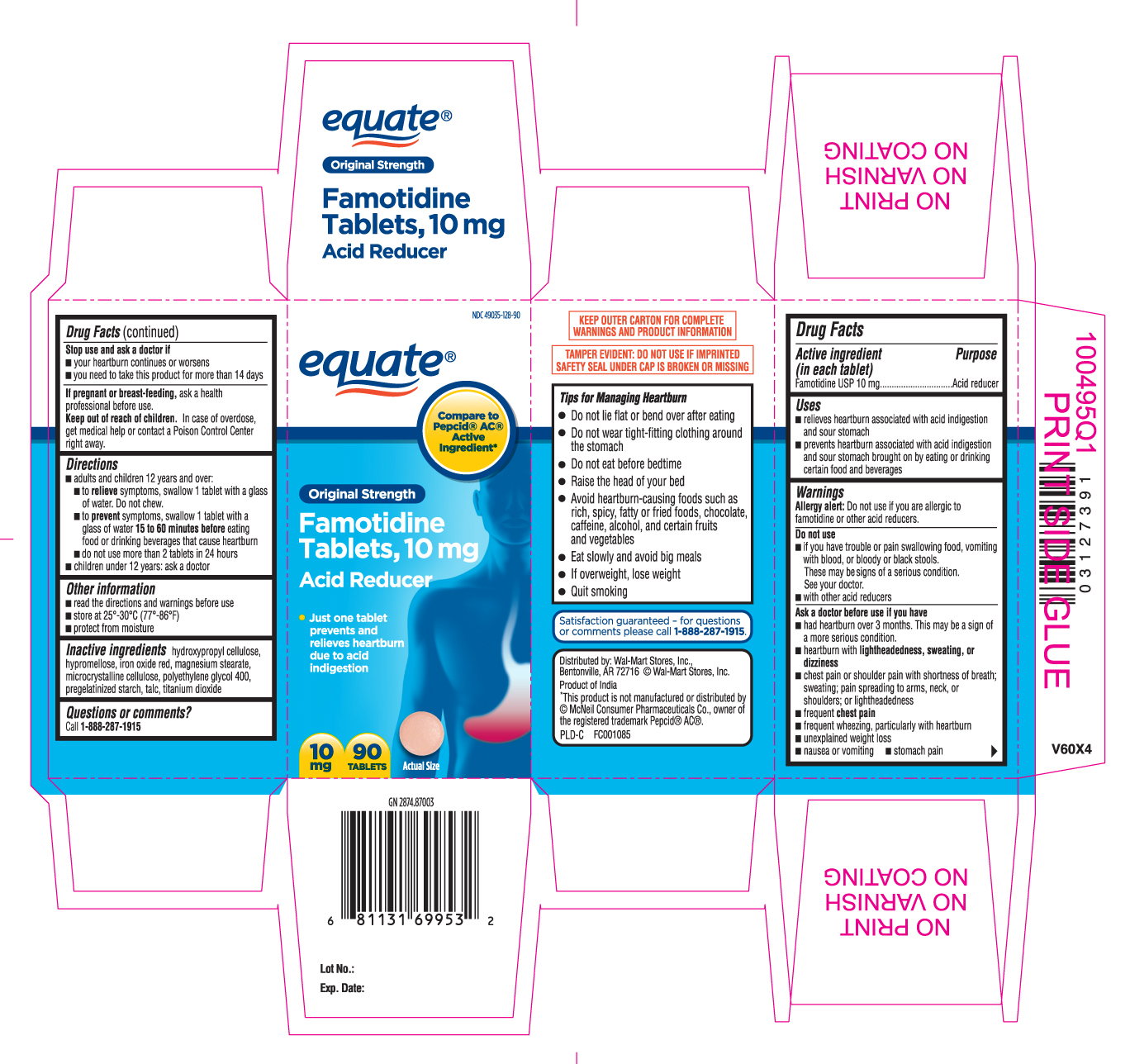
Drug Facts
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers.
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water 15 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
Other information
- read the directions and warnings before use
- store at 25°-30°C (77°-86°F)
- protect from moisture
Inactive ingredients
hydroxypropyl cellulose, hypromellose, iron oxide red, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, pregelatinized starch, talc, titanium dioxide
Principal Display Panel
Compare to Pepcid® AC® Active Ingredient*
Original Strength
Famotidine Tablets, 10 mg
Acid Reducer
- Just one tablet prevents and relieves heartburn due to acid indigestion
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Tips for Managing Heartburn
- Do not lie flat or bend over after eating
- Do not wear tight-fitting clothing around the stomach
- Do not eat before bedtime
- Raise the head of your bed
- Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
- Eat slowly and avoid big meals
- If overweight, lose weight
- Quit smoking
Distributed by: Wal-Mart Stores, Inc.
Bentonville, AR 72716 ©Wal-mart Stores, Inc.
Product of India
*This product is not manufactured or distributed by © McNeil Consumer Pharmaceuticals Co., owner of the registered trademark Pepcid® AC®.
| FAMOTIDINE
ORIGINAL STRENGTH
famotidine tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC) (051957769) |
| Registrant - P & L Development, LLC (079765031) |