Label: PREDNISOLONE ACETATE suspension/ drops
-
Contains inactivated NDC Code(s)
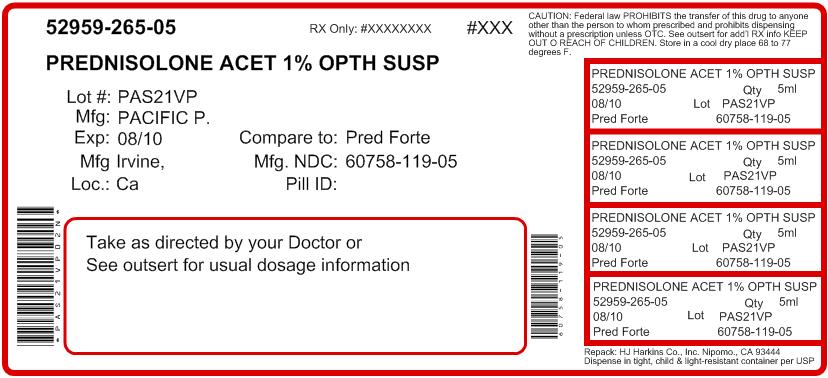
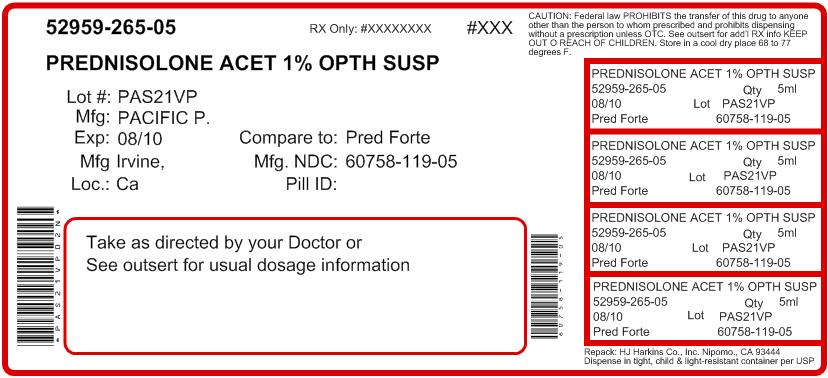
NDC Code(s): 52959-265-05, 52959-265-10, 52959-265-15 - Packager: H.J. Harkins Company, Inc.
- This is a repackaged label.
- Source NDC Code(s): 60758-119
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated November 17, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
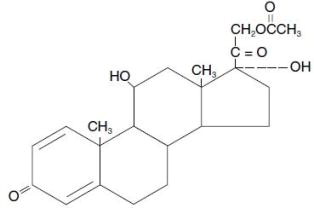
Prednisolone acetate ophthalmic suspension, USP 1.0% is a topical anti-inflammatory agent for ophthalmic use.
-
CLINICAL PHARMACOLOGY
Prednisolone acetate is a glucocorticoid that, on the basis of weight, has 3 to 5 times the anti-inflammatory potency of hydrocortisone. Glucocorticoids inhibit the edema, fibrin deposition, capillary dilation, and phagocytic migration of the acute inflammatory response, as well as capillary proliferation, deposition of collagen, and scar formation.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Prednisolone acetate ophthalmic suspension 1.0% is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. Prednisolone acetate ophthalmic suspension 1.0% is also contraindicated in individuals with known or suspected hypersensitivity to any of the ingredients of this preparation and to other corticosteroids.
-
WARNINGS
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Prolonged use may also suppress the host immune response and thus increase the hazard of secondary ocular infections.
Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation.
Acute purulent infections of the eye may be masked or activity enhanced by the presence of corticosteroid medication.
If this product is used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients. Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be checked frequently.
The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution; frequent slit lamp microscopy is recommended.
Corticosteroids are not effective in mustard gas keratitis and Sjögren's keratoconjunctivitis.
Contains sodium bisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
-
PRECAUTIONS
General: The initial prescription and renewal of the medication order beyond 20 milliliters of prednisolone acetate ophthalmic suspension 1.0% should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated.
As fungal infections of the cornea are particularly prone to develop coincidentally with longterm local corticosteroid applications, fungal invasion should be suspected in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal cultures should be taken when appropriate.
If this product is used for 10 days or longer, intraocular pressure should be monitored (see WARNINGS).
Information for patients: If inflammation or pain persists longer than 48 hours or becomes aggravated, the patient should be advised to discontinue use of the medication and consult a physician.
This product is sterile when packaged. To prevent contamination, care should be taken to avoid touching the bottle tip to eyelids or to any other surface. The use of this bottle by more than one person may spread infection. Keep bottle tightly closed when not in use. Keep out of the reach of children.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No studies have been conducted in animals or in humans to evaluate the potential of these effects.
Pregnancy Category C: Prednisolone has been shown to be teratogenic in mice when given in doses 1-10 times the human dose. There are no adequate well-controlled studies in pregnant women. Prednisolone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Dexamethasone, hydrocortisone, and prednisolone were ocularly applied to both eyes of pregnant mice five times per day on days 10 through 13 of gestation. A significant increase in the incidence of cleft palate was observed in the fetuses of the treated mice.
Nursing Mothers: It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Because of the potential for serious adverse reactions in nursing infants from prednisolone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Adverse reactions include, in decreasing order of frequency, elevation of intraocular pressure (IOP) with possible development of glaucoma and infrequent optic nerve damage, posterior subcapsular cataract formation, and delayed wound healing.
Although systemic effects are extremely uncommon, there have been rare occurrences of systemic hypercorticoidism after use of topical steroids.
Corticosteroid-containing preparations have also been reported to cause acute anterior uveitis and perforation of the globe. Keratitis, conjunctivitis, corneal ulcers, mydriasis, conjunctival hyperemia, loss of accommodation and ptosis have occasionally been reported following local use of corticosteroids.
The development of secondary ocular infection (bacterial, fungal, and viral) have occurred. Fungal and viral infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroid. The possibility of fungal invasion should be considered in any persistent corneal ulceration where steroid treatment has been used (see WARNINGS).
Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of prednisolone acetate ophthalmic suspension 1.0%. Other adverse events reported with the use of prednisolone acetate ophthalmic suspension 1.0% include: visual disturbance (blurry vision); foreign body sensation; and allergic reactions.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Shake well before using. Instill one to two drops into the conjunctival sac two to four times daily. During the initial 24 to 48 hours, the dosing frequency may be increased if necessary. Care should be taken not to discontinue therapy prematurely.
If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated (see PRECAUTIONS).
-
HOW SUPPLIED
Prednisolone acetate ophthalmic suspension, USP 1.0% is supplied sterile in opaque white LDPE plastic bottles with droppers with white high impact polystyrene (HIPS) caps as follows:
5mL in 10mL bottle - NDC 60758-119-05
10mL in 15mL bottle - NDC 60758-119-10
15mL in 15mL bottle - NDC 60758-119-15Note: Store at temperatures up to 25°C (77°F). Protect from freezing. Store in an upright position.
Rx only
Revised June 2004
© 2004 PACIFIC PHARMA
Irvine, CA 92612 U.S.A.
Printed in U.S.A.
6294X
71592PY10P
Repacked by:
H.J. Harkins Company, Inc.
513 Sandydale Drive
Nipomo, CA 93444 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREDNISOLONE ACETATE
prednisolone acetate suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52959-265(NDC:60758-119) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength prednisolone acetate (UNII: 8B2807733D) (prednisolone - UNII:9PHQ9Y1OLM) prednisolone acetate 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) boric acid (UNII: R57ZHV85D4) edetate disodium (UNII: 7FLD91C86K) hypromelloses (UNII: 3NXW29V3WO) polysorbate 80 (UNII: 6OZP39ZG8H) water (UNII: 059QF0KO0R) sodium bisulfite (UNII: TZX5469Z6I) sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-265-05 5 mL in 1 BOTTLE, DROPPER 2 NDC:52959-265-10 10 mL in 1 BOTTLE, DROPPER 3 NDC:52959-265-15 15 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA017011 08/19/1997 Labeler - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations Pacific Pharma, Inc. 877645267 MANUFACTURE