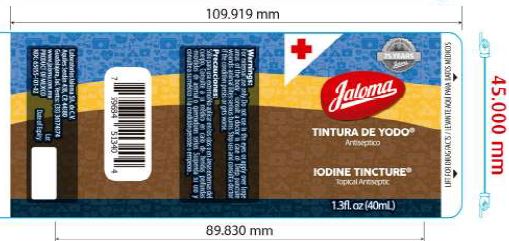
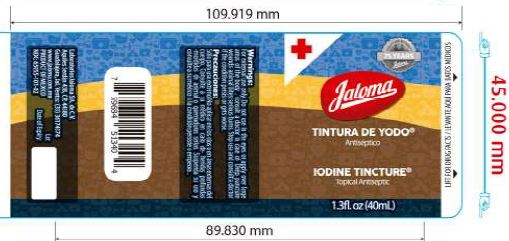
Label: IODINE TINCTURE tincture
- NDC Code(s): 65055-101-01, 65055-101-02
- Packager: Laboratorios Jaloma, S.A. de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
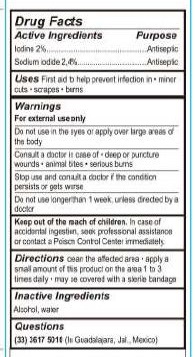
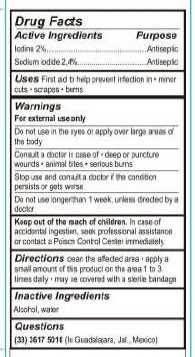
- Active Ingredients
- Purpose
- Uses
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- Inactive Ingredients
- QUESTIONS
- Iodine Tincture Topical Antiseptic
-
INGREDIENTS AND APPEARANCE
IODINE TINCTURE
iodine tincture tinctureProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65055-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 2 g in 100 mL SODIUM IODIDE (UNII: F5WR8N145C) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 2.4 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65055-101-01 25 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/24/2016 2 NDC:65055-101-02 40 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/24/2016 09/24/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 08/24/2016 Labeler - Laboratorios Jaloma, S.A. de C.V. (811122357) Establishment Name Address ID/FEI Business Operations Laboratorios Jaloma, S.A. de C.V. 811122357 manufacture(65055-101)