VAPRINO A-D- loperamide hydrochloride tablet
Boehringer Ingelheim Pharmaceuticals, Inc.
----------
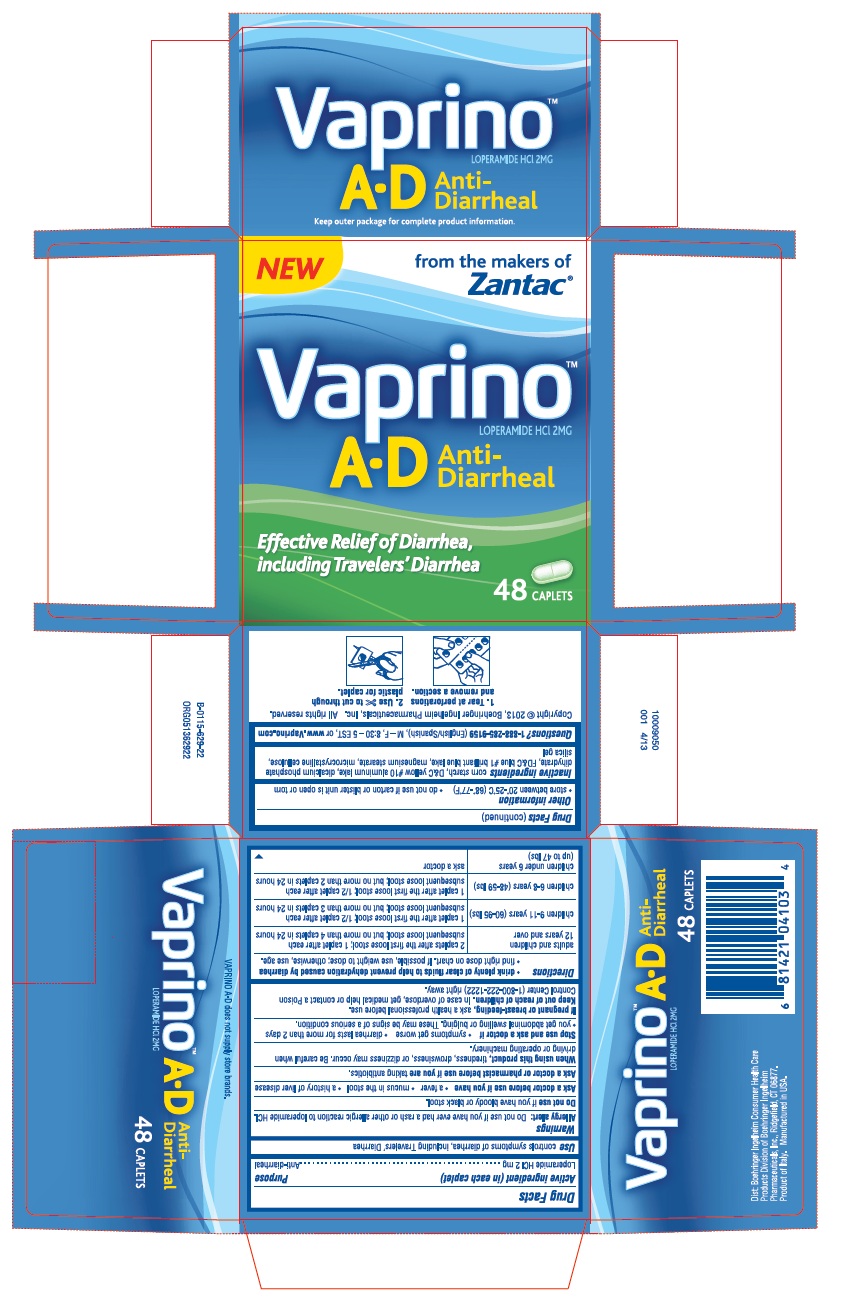
| Active ingredient (in each caplet) | Purpose |
| Loperamide HCl 2 mg .............................................................................................................................Anti-diarrheal | |
When using this product, tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
Stop use and ask a doctor if • symptoms get worse • diarrhea lasts for more than 2 days • you get abdominal swelling or bulging. These may be signs of a serious condition.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours | |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours | |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours | |
| children under 6 years (up to 47 lbs) | ask a doctor |
| VAPRINO A-D
loperamide hydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | MANUFACTURE(0597-0158) | |
Revised: 7/2017
Document Id: 77fb5f99-9c96-35c4-c4ae-fa3c28951536
Set id: c6cc4c7b-0c71-4c5a-37b9-731decaf2eb4
Version: 2
Effective Time: 20170723
Boehringer Ingelheim Pharmaceuticals, Inc.