Label: ADVANCED GEL HAND SANITIZER- alcohol solution
-
NDC Code(s):
47593-488-26,
47593-488-31,
47593-488-32,
47593-488-33, view more47593-488-41, 47593-488-49, 47593-488-56
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
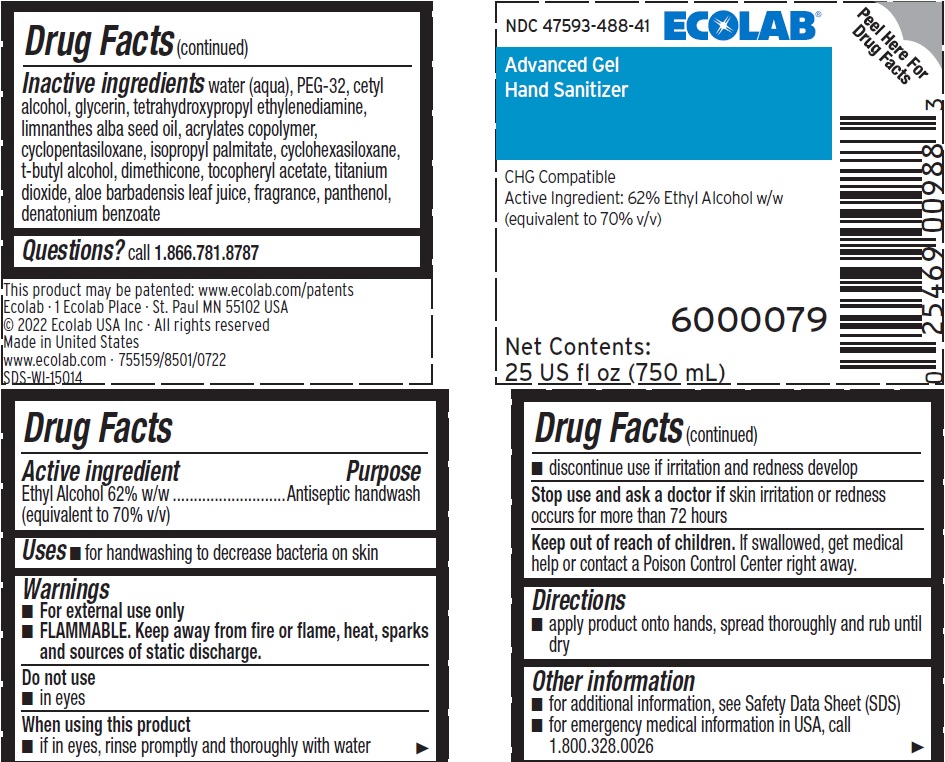
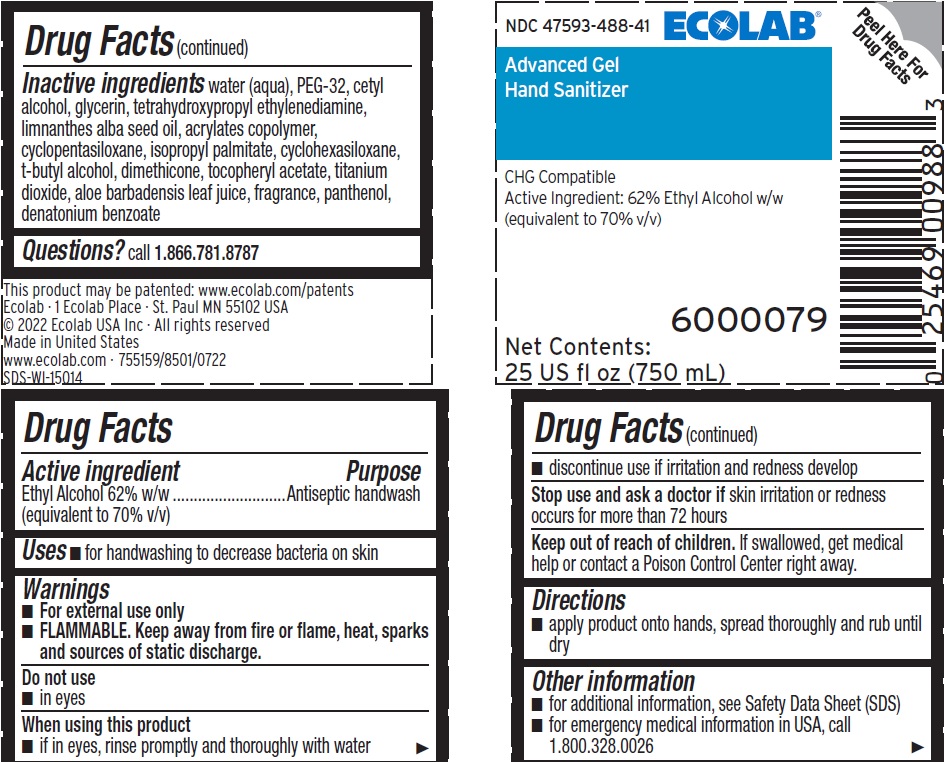
INACTIVE INGREDIENT
Inactive ingredients Water (Aqua), PEG-32, Cetyl Alcohol, Glycerin, Tetrahydroxypropyl Ethylenediamine, Limnanthes Alba Seed Oil , Acrylates Copolymer, Cyclopentasiloxane, Isopropyl Palmitate, Cyclohexasiloxane, t-Butyl Alcohol, Dimethicone, Tocopheryl Acetate, Titanium Dioxide, Aloe Barbadensis Leaf Juice, fragrance, Panthenol, Denatonium benzoate
- QUESTIONS
-
Representative label and principal display panel
NDC 47593-488-41 ECOLAB
Advanced Gel
Hand Sanitizer
CHG Compatible
Active Ingredient: 62% Ethyl Alcohol w/w
(equivalent to 70% v/v)
6000079
Net Contents:
25 US fl oz (750 mL)
This product may be patented: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA
© 2022 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com · 755159/8501/0722
SDS-WI-15014

-
INGREDIENTS AND APPEARANCE
ADVANCED GEL HAND SANITIZER
alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-488 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) EDETOL (UNII: Q4R969U9FR) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE (UNII: 92RU3N3Y1O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALOE VERA LEAF (UNII: ZY81Z83H0X) PANTHENOL (UNII: WV9CM0O67Z) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-488-33 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/30/2012 09/30/2024 2 NDC:47593-488-31 540 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/30/2012 3 NDC:47593-488-32 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/30/2012 11/24/2022 4 NDC:47593-488-26 1000 mL in 1 POUCH; Type 0: Not a Combination Product 11/30/2012 09/02/2024 5 NDC:47593-488-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/30/2012 09/30/2024 6 NDC:47593-488-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/30/2012 09/30/2024 7 NDC:47593-488-49 37 mL in 1 BOTTLE, PLASTIC; Type 1: Convenience Kit of Co-Package 11/30/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/30/2012 Labeler - Ecolab Inc. (006154611)