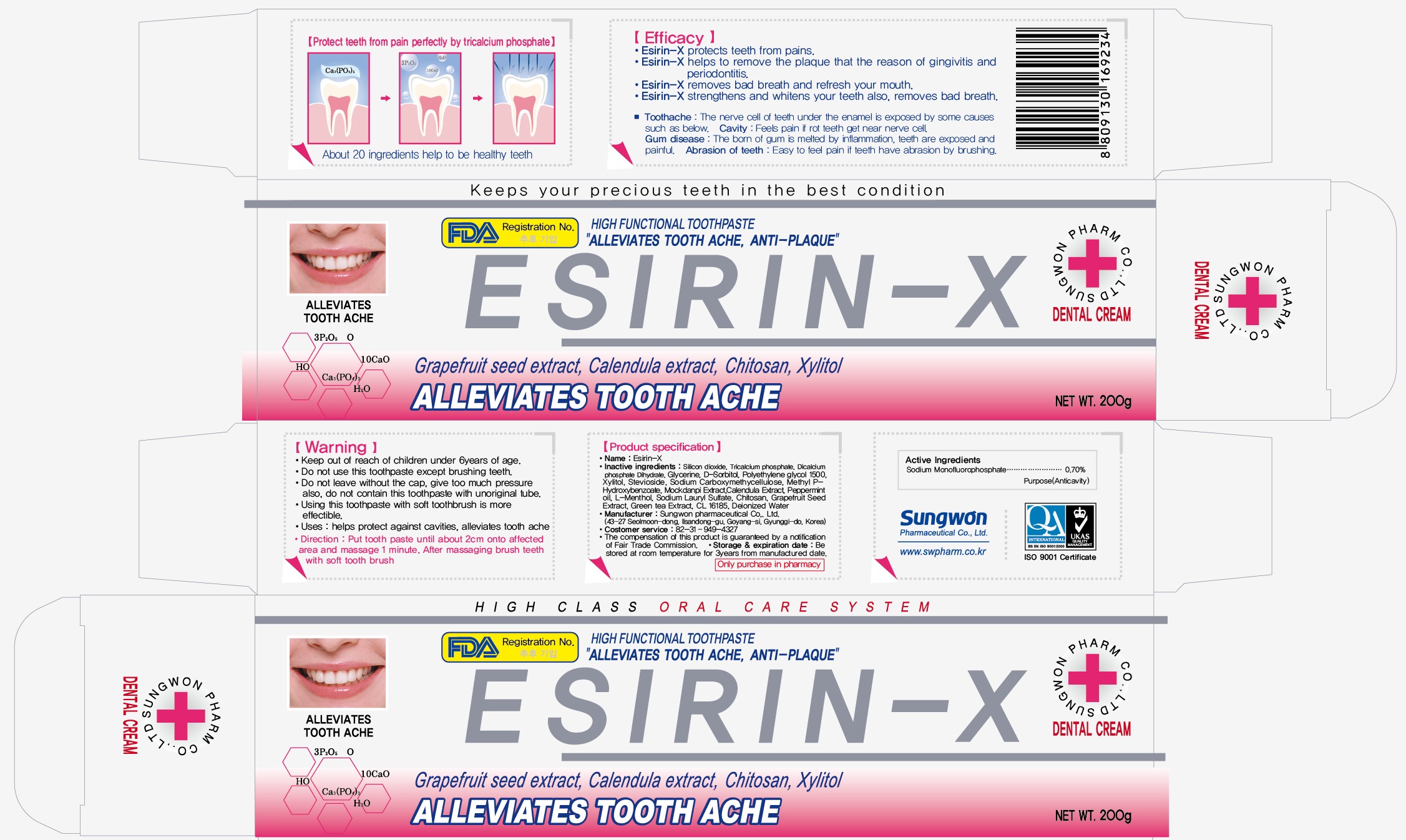
ESIRIN X- sodium monofluorophosphate paste
Sungwon Pharmaceutical Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Sodium Monofluorophosphate 0.7%
Silicon Dioxide
Tricalcium phosphate
Calcium phospahte, dibasic, dihydrate
Glycerin
Sorbitol
Polyehtylene glycol 1500
Xylitol
Stevioside
Carboxymethylcellulose sodium
methyl 3 Hydroxybenzoate
Mockdanpi Extract
Calendula officinalis flower
peppermint oil
L-menthol
Sodium lauryl sulfate
Chitosan
Grapefruit
Green tea leaf
CL 16185
Water
Keep out of Reach of Children.
Do not use this toothpaste except brushing teeth.
Do not leave without the cap, give too much pressure also.
Do not contain this toothpaste with unoriginal tube.
Helps protect against cavities, alleviates tooth ache
Put tooth paste until about 2 cm onto affected area and massage 1 minute. After massaging brush teeth with soft tooth brush.
Sungwon Pharmaceutical Co., Ltd.