MEBENDAZOLE- mebendazole tablet, chewable
Bryant Ranch Prepack
----------
MEBENDAZOLE TABLETS, USP 100 mg
9107
DESCRIPTION
Mebendazole is a (synthetic) broad-spectrum anthelmintic available as chewable tablets, each containing 100 mg of mebendazole. Inactive ingredients are: anhydrous lactose NF, corn starch, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, sodium saccharin, sodium starch glycolate, stearic acid, and FD&C Yellow #6.
Mebendazole is methyl 5-benzoylbenzimidazole-2-carbamate and has the following structural formula:
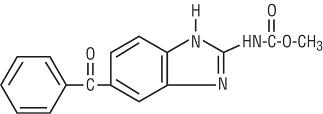
Molecular Formula: C16H13N3O3
Mebendazole is a white to slightly yellow powder with a molecular weight of 295.29. It is less than 0.05% soluble in water, dilute mineral acid solutions, alcohol, ether and chloroform, but is soluble in formic acid.
CLINICAL PHARMACOLOGY
Following administration of 100 mg twice daily for three consecutive days, plasma levels of mebendazole and its primary metabolite, the 2-amine, do not exceed 0.03 mcg/mL and 0.09 mcg/mL, respectively. All metabolites are devoid of anthelmintic activity. In man, approximately 2% of administered mebendazole is excreted in urine and the remainder in the feces as unchanged drug or a primary metabolite.
INDICATIONS AND USAGE
Mebendazole tablets are indicated for the treatment of Enterobius vermicularis (pinworm), Trichuris trichiura (whipworm), Ascaris lumbricoides (common roundworm), Ancylostoma duodenale (common hookworm), Necator americanus (American hookworm) in single or mixed infections.
Efficacy varies as a function of such factors as preexisting diarrhea and gastrointestinal transit time, degree of infection, and helminth strains. Efficacy rates derived from various studies are shown in the table below:
| Pinworm (enterobiasis) | Whipworm (trichuriasis) | Common Roundworm (ascariasis) | Hookworm | |
| Cure rates mean | 95% | 68% | 98% | 96% |
| Egg reduction mean | — | 93% | 99% | 99% |
CONTRAINDICATIONS
Mebendazole is contraindicated in persons who have shown hypersensitivity to the drug.
WARNINGS
There is no evidence that mebendazole, even at high doses, is effective for hydatid disease. There have been rare reports of neutropenia and agranulocytosis when mebendazole was taken for prolonged periods and at dosages substantially above those recommended.
PRECAUTIONS
General
Periodic assessment of organ system functions, including hematopoietic and hepatic, is advisable during prolonged therapy.
Information for Patients
Patients should be informed of the potential risk to the fetus in women taking mebendazole during pregnancy, especially during the first trimester (See Pregnancy).
Patients should also be informed that cleanliness is important to prevent reinfection and transmission of the infection.
Drug Interactions
Preliminary evidence suggests that cimetidine inhibits mebendazole metabolism and may result in an increase in plasma concentrations of mebendazole.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In carcinogenicity tests of mebendazole in mice and rats, no carcinogenic effects were seen at doses as high as 40 mg/kg (one to two times the human dose, based on mg/m2) given daily over two years. Dominant lethal mutation tests in mice showed no mutagenicity at single doses as high as 640 mg/kg (18 times the human dose, based on mg/m2). Neither the spermatocyte test, the F1 translocation test, nor the Ames test indicated mutagenic properties. Doses up to 40 mg/kg in mice (equal to the human dose, based on mg/m2), given to males for 60 days and to females for 14 days prior to gestation, had no effect upon fetuses and offspring, though there was slight maternal toxicity.
Pregnancy
Teratogenic Effects
Pregnancy category C
Mebendazole has shown embryotoxic and teratogenic activity in pregnant rats at single oral doses as low as 10 mg/kg (approximately equal to the human dose, based on mg/m2). In view of these findings the use of mebendazole is not recommended in pregnant women. Although there are no adequate and well-controlled studies in pregnant women, a postmarketing survey has been done of a limited number of women who inadvertently had consumed mebendazole during the first trimester of pregnancy. The incidence of spontaneous abortion and malformation did not exceed that in the general population. In 170 deliveries on term, no teratogenic risk of mebendazole was identified.
ADVERSE REACTIONS
Gastrointestinal
Transient symptoms of abdominal pain and diarrhea in cases of massive infection and expulsion of worms.
OVERDOSAGE
In the event of accidental overdosage, gastrointestinal complaints lasting up to a few hours may occur. Vomiting and purging should be induced.
DOSAGE AND ADMINISTRATION
The same dosage schedule applies to children and adults. The tablet may be chewed, swallowed, or crushed and mixed with food.
| Pinworm (enterobiasis) | Whipworm (trichuriasis) | Common Roundworm (ascariasis) | Hookworm | |
| Dose | 1 tablet, once | 1 tablet morning and evening for 3 consecutive days | 1 tablet morning and evening for 3 consecutive days | 1 tablet morning and evening for 3 consecutive days |
If the patient is not cured three weeks after treatment, a second course of treatment is advised. No special procedures, such as fasting or purging, are required.
MEBENDAZOLE TABLETS, USP
CHEWABLE TABLET
100 mg
Your doctor has prescribed this medicine to treat an infection caused by an intestinal worm. Follow your doctor’s instructions carefully. In addition to your doctor’s treatment, you can help prevent reinfection and infection of other people by understanding a few simple facts about worms.
PINWORM: Pinworms look like tiny white threads and live in the bowel. Usually at night, they travel to the rectal opening and lay eggs on the outside skin. This sometimes causes itching which may be very annoying. That is why restless sleep is a frequent sign of pinworms, especially in children. Scratching will cause pinworm eggs to stick to the fingers. Reinfection will result if the fingers are placed in the mouth.
The eggs, which are too small to see, contaminate whatever they come in contact with: bedclothes, underwear, hands, and food touched by contaminated hands. Even eggs floating in the air can be swallowed and cause infection. Pinworms are highly contagious. Even the cleanest and most careful people can get them.
To help prevent reinfection follow these rules:
- Wash hands and fingernails with soap often during the day, especially before eating and after using the toilet.
- Wear tight underpants both day and night. Change them daily.
- For several days after treatment, clean the bedroom floor by vacuuming or damp mopping. Avoid dry sweeping that may stir up dust.
- After treatment, wash bed linens and night clothes (don’t shake them).
- Keep the toilet seats clean
HOOKWORM, WHIPWORM AND ROUNDWORM: These worms also live in the bowel.
Eggs from the worms are deposited in the soil if an infected person fails to use a toilet or bathroom. Since the eggs can live only in warm soil, they are found most often where the soil never freezes in winter. People living or traveling in areas with warm winters may have these infections. The eggs in the soil are usually carried to the mouth on food or by contact with dirty hands. In the case of hookworms a pre-adult form of the worm actually penetrates the skin (usually the foot) and burrows its way into the bloodstream. Once inside the body, they grow and breed inside the bowel. New eggs are released in the feces.
Therefore, poor sewage disposal or the use of human waste for fertilizer can contaminate the ground with new eggs, which can then reinfect people.
The medication used to treat these worms causes them to be expelled from the body. Hookworms and whipworms may be seen and resemble small white threads. Roundworms are much larger and easily seen.
To help prevent reinfection follow these rules:
- Wash hands and fingernails with soap often during the day, especially before eating and after using the toilet.
- Wash all fruits and vegetables thoroughly or cook them well.
- Wear shoes.
- Use the bathroom.
Follow your doctor’s advice, take the medication he gives you and follow the rules mentioned here. If you have other questions about worms, be sure to ask your doctor.
WARNING: Do not take this medication if you are pregnant or think you may be pregnant. Consult your physician.
Manufactured By:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. D 9/2007
| MEBENDAZOLE
mebendazole tablet, chewable |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-1528) , RELABEL(63629-1528) | |
