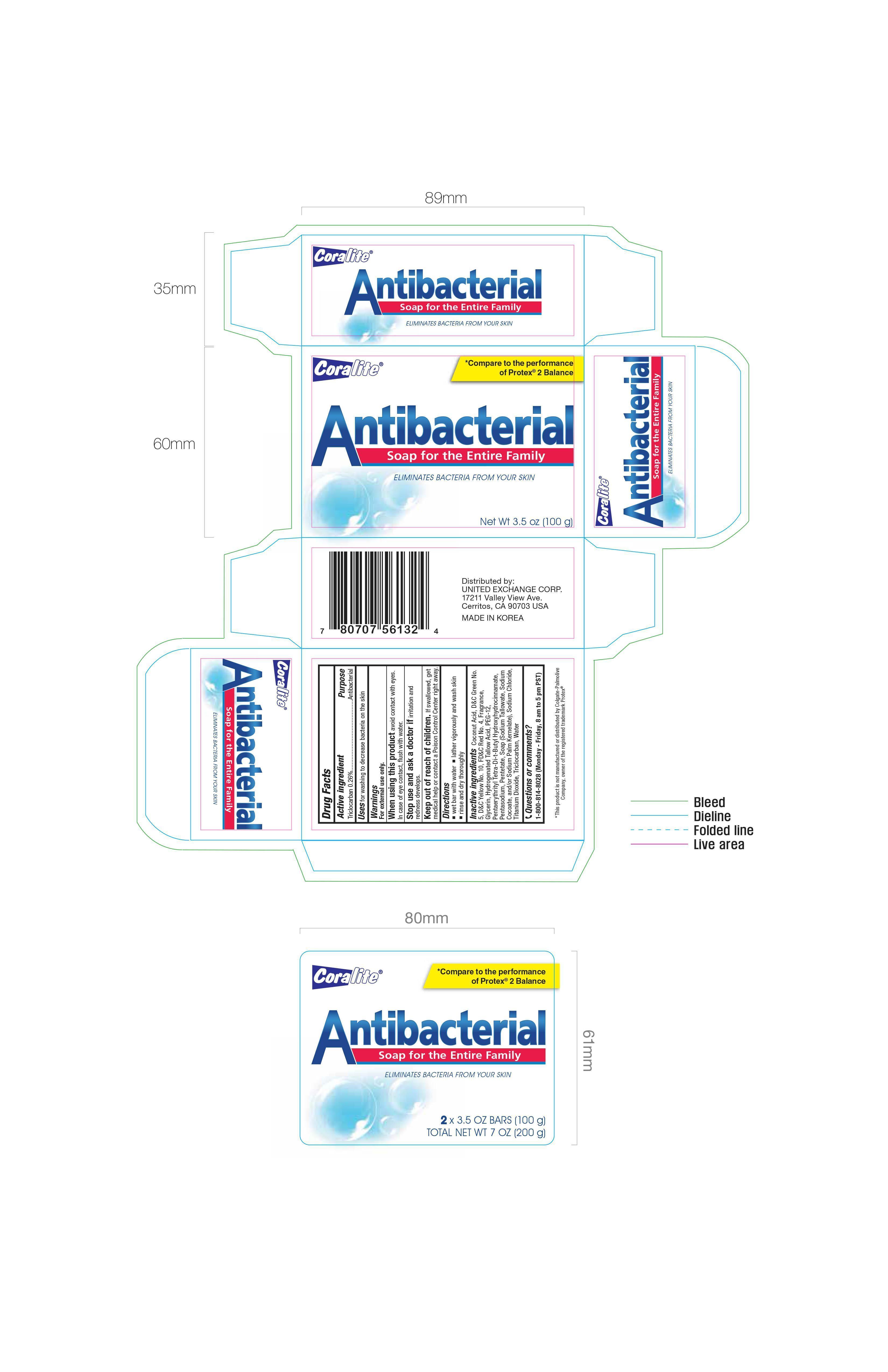
CORALITE ANTIBACTERIAL- triclocarban soap
United Exchange Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients Coconut Acid, D&C Green No. 5, D&C Yellow No. 10, FD&C Red No. 4, Fragrance, Glycerin, Hydrogenated Tallow Acid, PEG-12, Pentaerythrityl Tetra-Di-t-Butyl Hydroxyhydrocinnamate, Pentasodium, Pentetate, Soap (Sodium Tallowate, Sodium Coconate, and/or Sodium Palm Kernelate), Sodium Chloride, Titanium Dioxide, Triclocarban, Water
| CORALITE ANTIBACTERIAL
triclocarban soap |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - United Exchange Corp (840130579) |
Revised: 7/2017
Document Id: 2378ecaa-88bb-46bf-8883-004f2320a84b
Set id: c30a814b-f343-48cb-975b-6bb2e4dcf34b
Version: 2
Effective Time: 20170705
United Exchange Corp