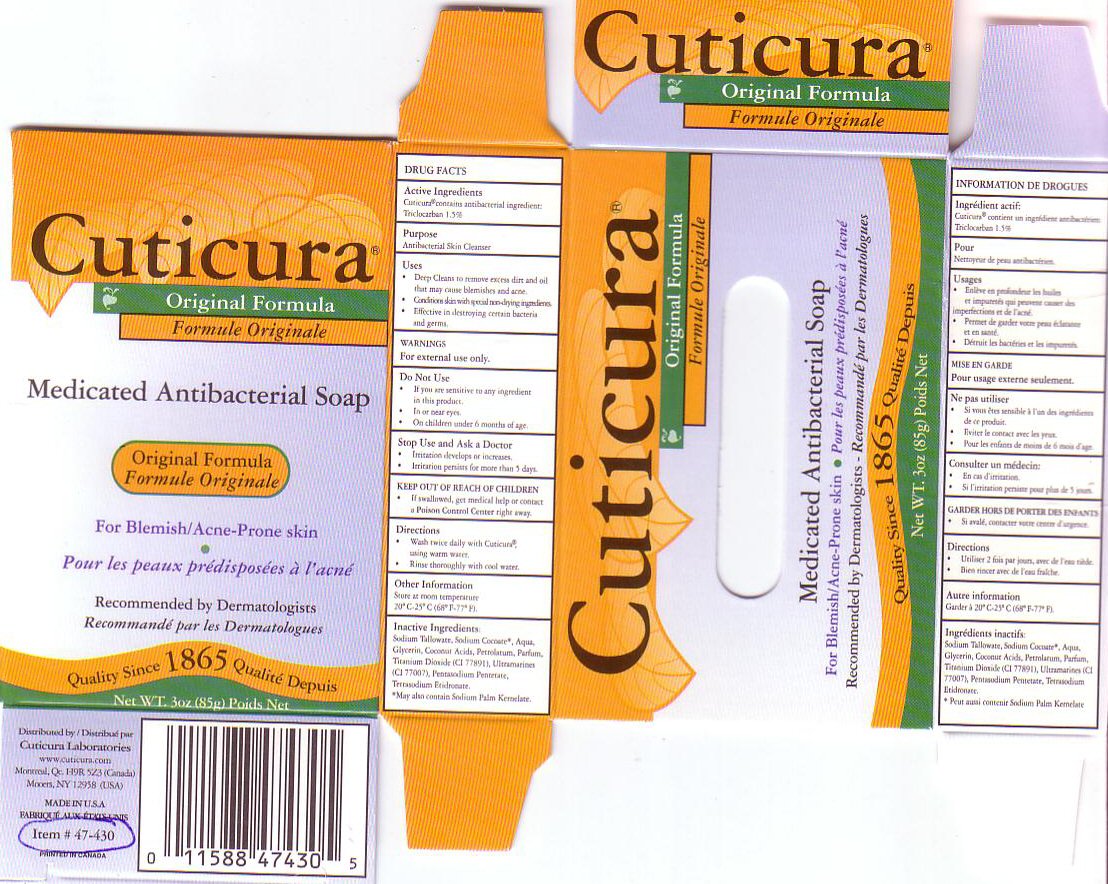
CUTICURA MEDICATED ANTIBACTERIAL BAR- triclocarban soap
Bradford Soap Works, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cuticure - 3000
USES
* DEEP CLEANS TO REMOVE EXCESS DIRT AND OIL THAT MAY CAUSE BLEMISHES AND ACNE.
* CONDITIONS SKIN WITH SPECIAL NON-DRYING INGREDIENTS.
* EFFECTIVE IN DESTROYING CERTAIN BACTERIA AND GERMS.
DO NOT USE
- IF YOU ARE SENSITIVE TO ANY INGREDIENTS IN THIS PRODUCT.
- IN OR NEAR EYES
- ON CHILDREN UNDER 6 MONTHS OF AGE.
STOP USE AND ASK A DOCTOR
- IRRITATION DEVELOPS OR INCREASES.
- IRRITATION PERSISTS FOR MORE THAN 5 DAYS.
KEEP OUT OF REACH OF CHILDREN
IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
| CUTICURA MEDICATED ANTIBACTERIAL BAR
triclocarban soap |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bradford Soap Works, Inc. (001201045) |
| Registrant - Bradford Soap Works, Inc. (001201045) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc. | 001201045 | manufacture(11118-3000) , label(11118-3000) | |
Revised: 7/2017
Document Id: a2a3b926-e4d9-4212-9828-5445b013515b
Set id: c3015a64-0bd4-47ee-bdc9-1c42712567e4
Version: 3
Effective Time: 20170706
Bradford Soap Works, Inc.