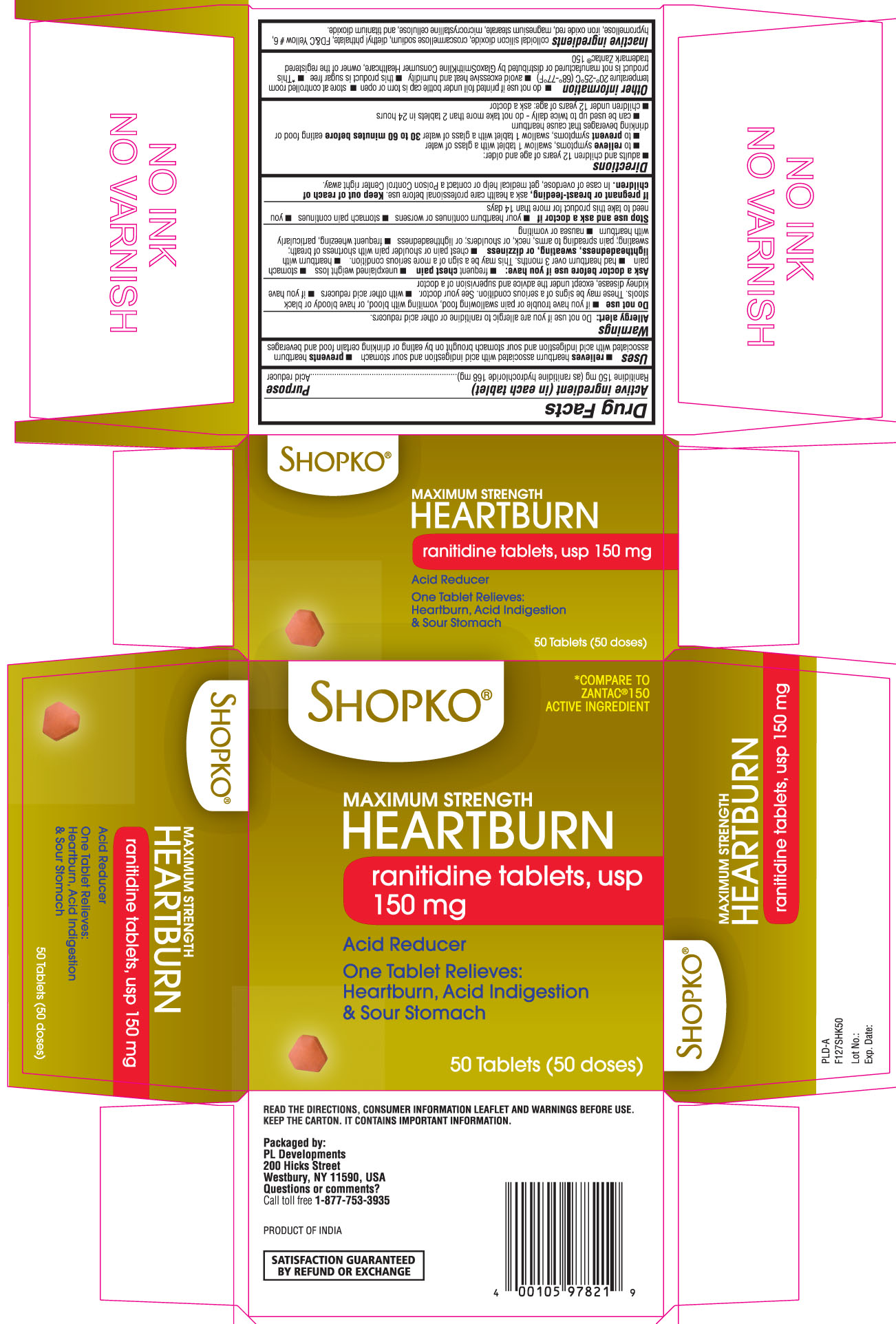
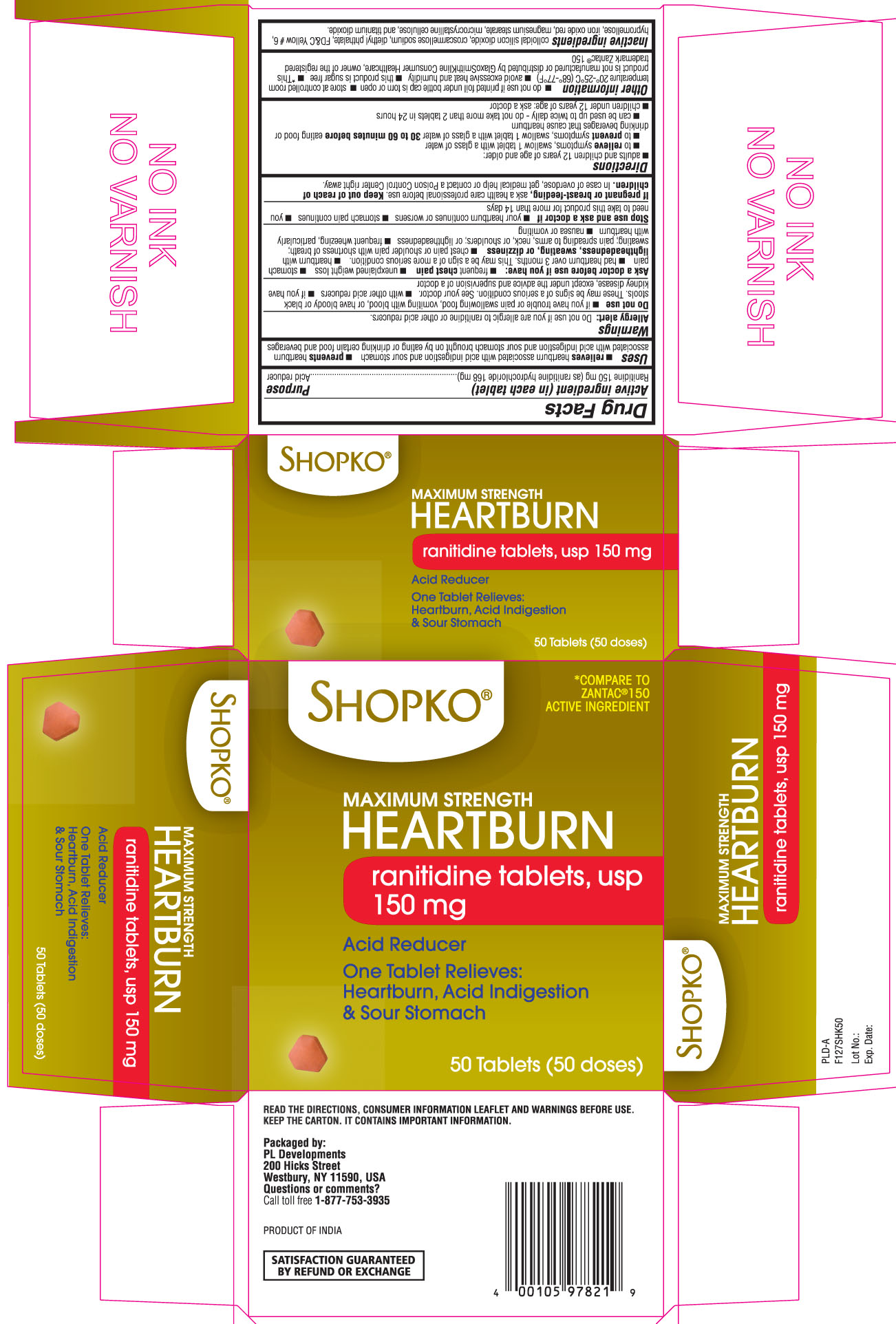
Label: RANITIDINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 37012-127-50 - Packager: Shopko Stores Operating Co., LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 29, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- Do not use
-
Ask a doctor before use if you have:
- frequent chest pain
- unexplained weight loss
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent wheezing, particularly with heartburn
- nausea or vomiting
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- adults and children 12 years and older:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily -do not take more than 2 tablets in 24 hours
- children under 12 years of age: ask a doctor
- adults and children 12 years and older:
-
Other information
- do not use if printed foil under cap is torn or open
- store at controlled room temperature 20°-25°C (68°-77°F)
- avoid excessive heat or humidity
- this product is sugar free
- *This product is not manufactured or distributed by Glaxosmithkline Consumer Healthcare, owner of the registered trademark Zantac® 150
- Inactive Ingredients
-
Principal display panel
SHOPKO®
Maximum Strength HEARTBURN
Ranitidine Tablets, USP 150 mg
Acid Reducer
One tablet relieves: heartburn, acid indigestion & sour stomach
*Compare to Zantac 150® active ingredient
Read the directions, consumer information leaflet and warnings before use. Keep the carton. It contains important information.
Packaged by:
PL Developments
200 Hicks street
Westbury, NY 11590, USA
Questions or comments?
call toll free 1-877-753-3935
Product of INDIA
- Product Label
-
INGREDIENTS AND APPEARANCE
RANITIDINE
ranitidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37012-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIETHYL PHTHALATE (UNII: UF064M00AF) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape HEXAGON (6 SIDED) Size 10mm Flavor Imprint Code W;741 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37012-127-50 1 in 1 CARTON 1 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078653 07/11/2010 Labeler - Shopko Stores Operating Co., LLC. (023252638)