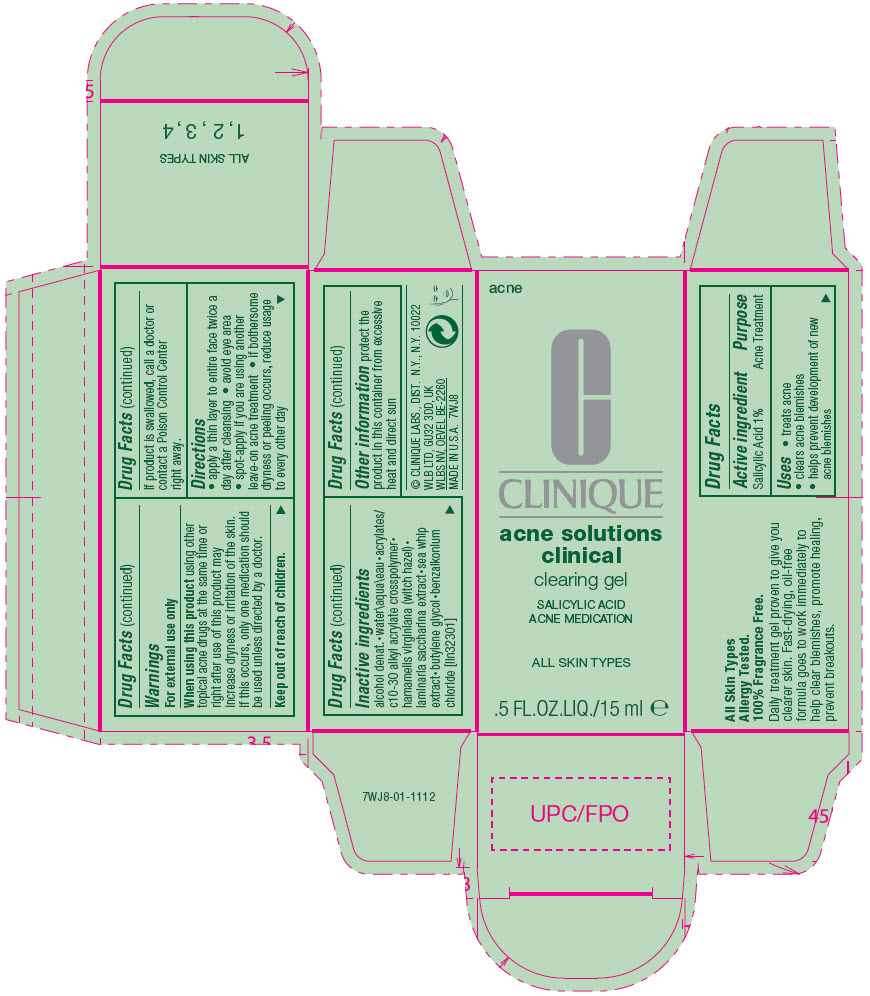
Active ingredient
Salicylic Acid 1%
Uses
- treats acne
- clears acne blemishes
- helps prevent development of new acne blemishes
Warnings
For external use only
When using this product using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Keep out of reach of children.
If product is swallowed, call a doctor or contact a Poison Control Center right away.
Directions
- apply a thin layer to entire face twice a day after cleansing
- avoid eye area
- spot-apply if you are using another leave-on acne treatment
- if bothersome dryness or peeling occurs, reduce usage to every other day
Inactive ingredients
alcohol denat.•water\aqua\eau•acrylates/c10-30 alkyl acrylate crosspolymer•hamamelis virginiana (witch hazel)•laminaria saccharina extract•sea whip extract•butylene glycol•benzalkonium chloride [iln32301]
Other information
protect the product in this container from excessive heat and direct sun
PRINCIPAL DISPLAY PANEL - 15 ml Tube Carton
acne
CLINIQUE
acne solutions
clinical
clearing gel
SALICYLIC ACID
ACNE MEDICATION
ALL SKIN TYPES
.5 FL.OZ.LIQ./15 ml e