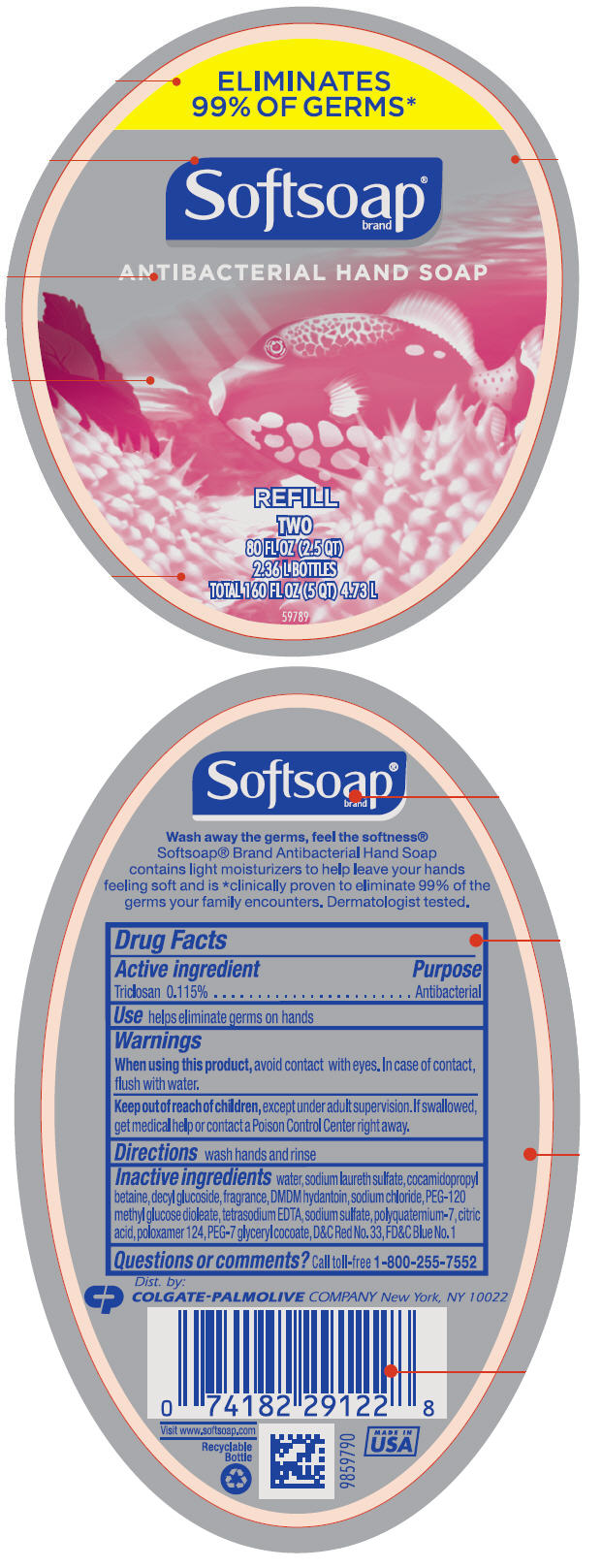
SOFTSOAP ANTIBACTERIAL HAND- triclosan liquid
Colgate-Palmolive Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Softsoap®
brand
ANTIBACTERIAL HAND SOAP
REFILL
Inactive ingredients
water, sodium laureth sulfate, cocamidopropyl betaine, decyl glucoside, fragrance, DMDM hydantoin, sodium chloride, PEG-120 methyl glucose dioleate, tetrasodium EDTA, sodium sulfate, polyquaternium-7, citric acid, poloxamer 124, PEG-7 glyceryl cocoate, D&C Red No. 33, FD&C Blue No. 1
| SOFTSOAP
ANTIBACTERIAL HAND
triclosan liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Company (068693167) |
Revised: 12/2017
Document Id: 7cd68a67-2bb5-483d-8fe7-5be9a2e8ac05
Set id: bfefb15d-c659-43ea-96f3-fc25ecbf9167
Version: 4
Effective Time: 20171201
Colgate-Palmolive Company