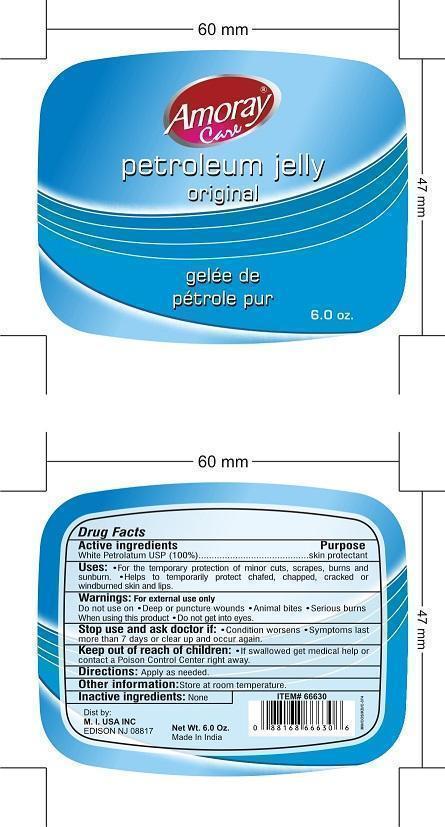
AMORAY CARE PETROLEUM ORIGINAL- petrolatum jelly
MY IMPORT USA INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
- FOR TEMPORARY PROTECTION OF MINOR CUTS, SCRAPES, BURNS AND SUNBURN.
- HELPS TO TEMPORARILY PROTECT CHAFED, CHAPPED, CRACKED OR WINDBURNED SKIN AND LIPS.
Keep Out of Reach of Children
- Children if swallowed get medical help or contact Poison Control Center right away.
| AMORAY CARE PETROLEUM
ORIGINAL
petrolatum jelly |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - MY IMPORT USA INC (195767988) |
Revised: 2/2017
Document Id: 57484dbb-304a-465f-949f-55a43b213a4c
Set id: bfa33a6f-748a-4d0a-90b7-ce87f241870c
Version: 7
Effective Time: 20170211
MY IMPORT USA INC