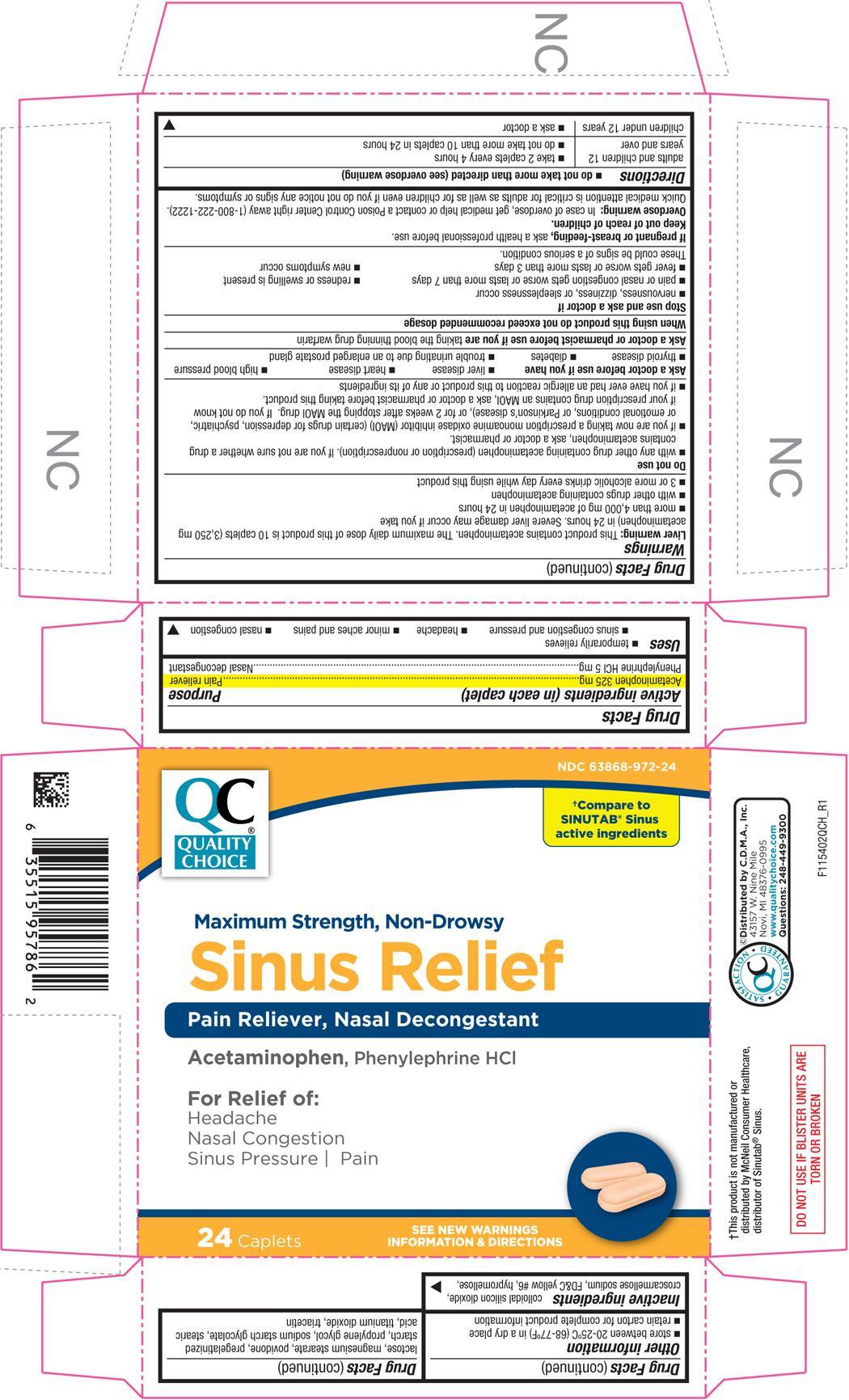
SINUS RELIEF- acetaminophen and phenylephrine hydrochloride tablet, coated
Chain Drug Marketing Association
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
QCH - 1154 - 2019-1007
Uses
- temporarily relieves
- sinus congestion and pressure
- headache
- minor aches and pains
- nasal congestion
Warnings
Liver warning
This product contains acetaminophen. The maximum daily dose of this product is 10 caplets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
Other information
- store between 20°-25°C (68°-77°F) in a dry place
- retain carton for complete product information
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, FD&C yellow #6, hypromellose, lactose, magnesium stearate, povidone, pregelatinized starch, propylene glycol, sodium starch glycolate, stearic acid, titanium dioxide, triacetin
PRINCIPAL DISPLAY PANEL
NDC 63868-972-24
QUALITY CHOICE
†Compare to SINUTAB® Sinus active ingredients
Maximum Strength, Non-Drowsy
Sinus Relief
Pain Reliever, Nasal Decongestant
Acetaminophen, Phenylephrine HCl
For Relief of:
Headache
Nasal Congestion
Sinus Pressure / Pain
24 Caplets
SEE NEW WARNINGS INFORMATION & DIRECTIONS
| SINUS RELIEF
acetaminophen and phenylephrine hydrochloride tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Chain Drug Marketing Association (011920774) |