DOCUSATE SODIUM STOOL SOFTENER- docusate sodium capsule, liquid filled
Robinson Pharma, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
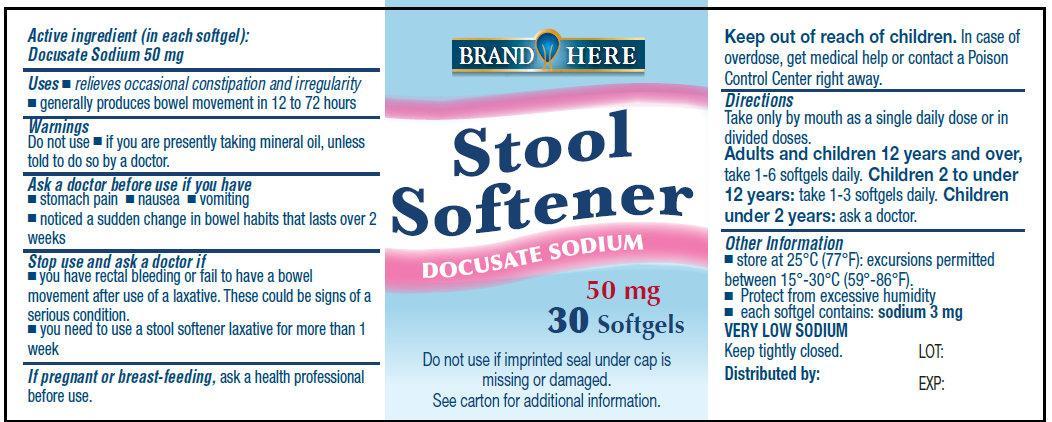
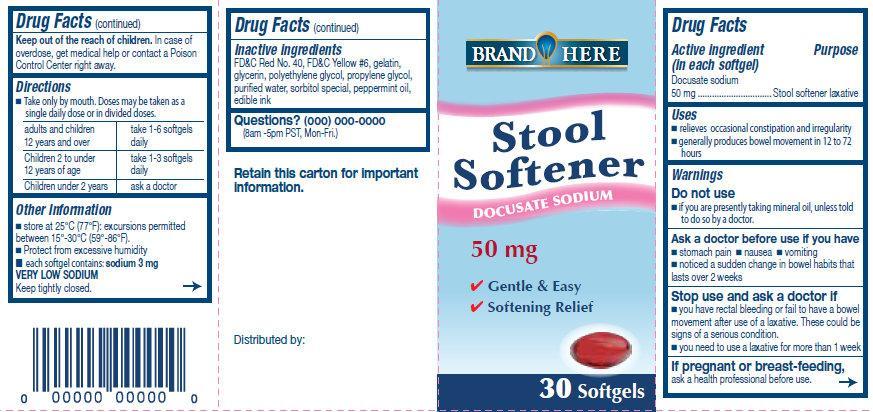
Docusate Sodium Stool Softener
Uses
- relieves occasional constipation and irregularity
- generally produces bowel movement in 12 to 72 hours
Warnings
Do not use
- if you are presently taking mineral oil, unless told to do by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding, ask a health professional before use.
Directions
- Take only by mouth. Doses may be taken as a single does or in divided doses.
| adults and children 12 years and over
| take 1-6 softgels
|
| Children 2 to under 12 years of age
| take 1-3 softgels
|
| Children under 2 years
| ask a doctor
|
Other Information
- store at 25 degrees C (77 degrees F); excursions permitted between 15 degrees - 30 degrees C (59 degrees - 86 degrees F).
- Protect from excessive humidity
- each softgel contains : sodium 3 mg
VERY LOW SODIUM
Keep tightly closed.
| DOCUSATE SODIUM STOOL SOFTENER
docusate sodium capsule, liquid filled |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Robinson Pharma, Inc. (831560578) |
| Registrant - Robinson Pharma, Inc. (831560578) |
Revised: 1/2017
Document Id: 4765d329-ea6b-4d20-e054-00144ff8d46c
Set id: be61a66c-75f4-4ae9-84e2-c995de8e26bb
Version: 2
Effective Time: 20170131
Robinson Pharma, Inc.